Single-Fluorescent Protein Biosensors
About the Innovation
Download our Reagents Catalog here.
Maltose Sensor - Glutamate Sensor - Phosphonate Sensor
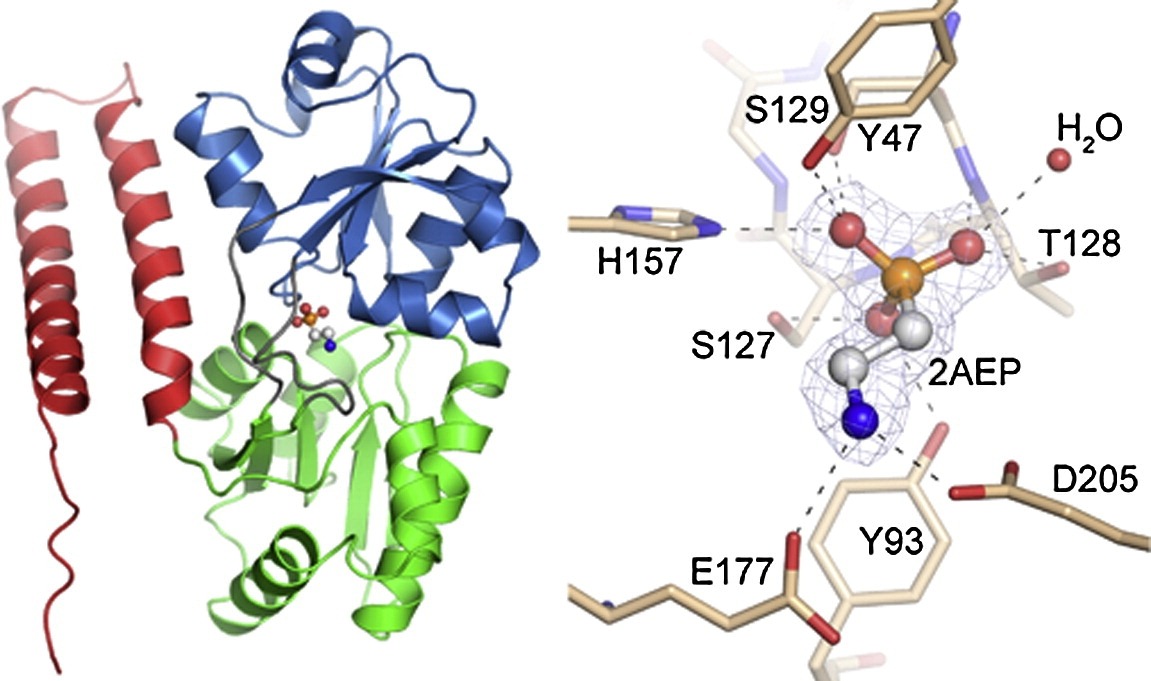
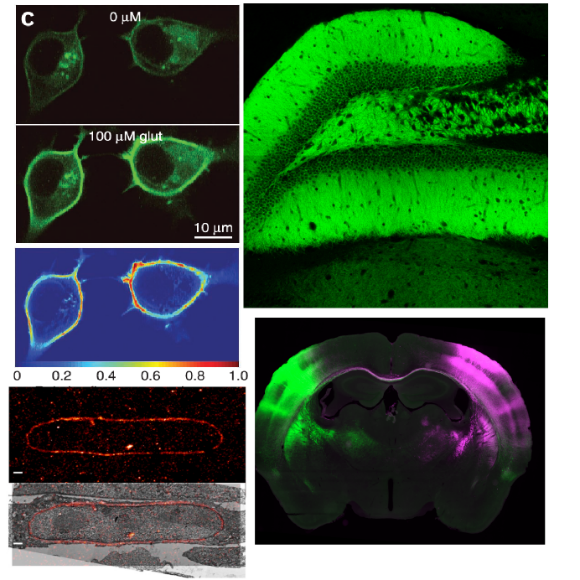
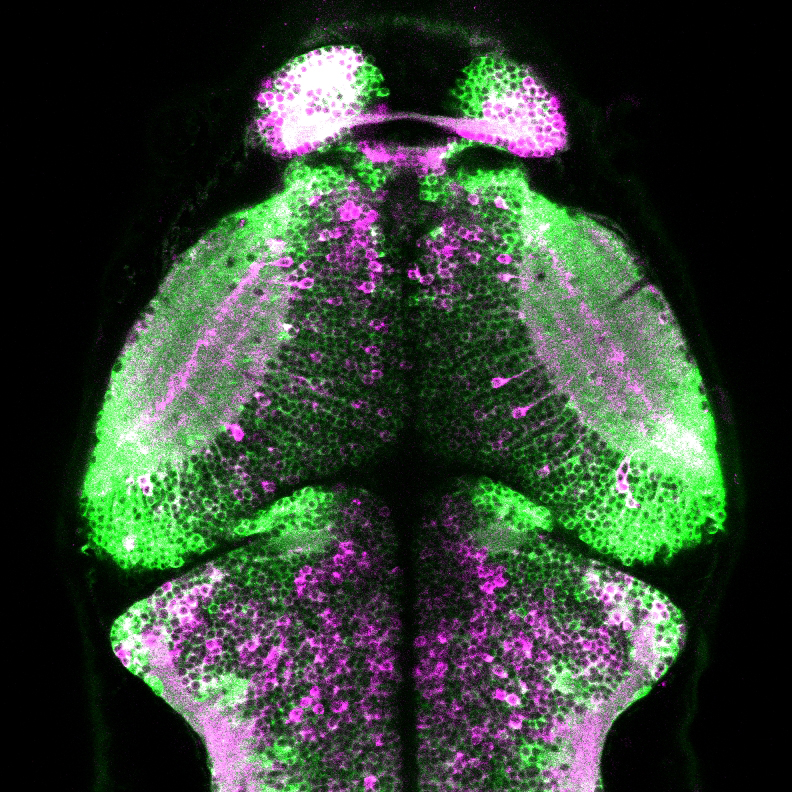
Inventors at Howard Hughes Medical Center’s Janelia Research Campus have produced a general strategy for the creation of genetically encoded intensity‐based fluorescent sensors from the bacterial periplasmic binding protein superfamily and a subsequent unique set of analytical tools, or sensors, that can measure the presence of a single molecular analyte in a complex mixture using standard optical technology. The binding of a targeted analyte to the permuted fluorescent protein induces a dramatic conformational change in the reporter molecule and a change in the molecule’s fluorescent protein properties, detectable using generic fluorescent detector instrumentation. The approach has been demonstrated by engineering a four‐color family of maltose indicators from Escherichia coli maltodextrin‐binding protein. The sensors were iteratively optimized to be bright and have large fluorescent increases upon maltose-binding, under both 1‐ and 2‐photon illumination. Sensors have been developed that demonstrate rapid maltose transport across the plasma membrane of individual E. coli bacteria. The indicators will likely be similarly useful in eukaryotic cells and intact organisms. The periplasmic binding protein superfamily includes scaffolds specific for several analytes whose visualization would be critical to the reverse engineering of complex systems such as neural networks, biosynthetic pathways, and signal transduction cascades.
The value of the technology has been demonstrated in preparation of multiple sensors of periplasmic binding protein and analyte targets, including:
- Maltosetriose and starches from a thermophilic maltotriose‐binding protein, glutamate from an E. coli glutamate‐binding protein, phosphonates, and an E. coli phosphonate‐binding protein.
Reagent Data:
Addgene: pCMV.iGluSnFR (41732) and pRSET.GltI253-cpGFP.L1LV/L2NP (41733). AAV virus also available; see Online Methods.
Advantages:
- A general method for constructing intensity-based biosensors.
- The periplasmic binding protein superfamily provides immense ligand-binding diversity.
- Tunable binding site affinity and specificity.
- Detection using standard optical technologies.
Applications:
- Study of sugar metabolism in agriculture-based products and potential biofuels.
- Neurobiological studies of neurotransmitters such as glutamate.
- Detection of synthetic phosphonates present in herbicides, antibiotics, household detergents, insecticides, and chemical warfare agents.
- Blood glucose monitoring for diabetes.
Opportunity:
Free for Non-Profit Research and available for Commercial License
Patent Protection:
U.S. Patents 9,719,992, 9,939,437, 10,060,920 and 10,345,297
For inquiries, please reference:
Janelia 2010-002