Main Menu (Mobile)- Block
- Overview
-
Support Teams
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- High Performance Computing
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Stem Cell & Primary Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing
- Viral Tools
- Vivarium
- Open Science
- You + Janelia
- About Us
Main Menu - Block
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- High Performance Computing
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Stem Cell & Primary Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing
- Viral Tools
- Vivarium
What We Do
The Electron Microscopy Shared Resource (EMSR) enables biological research at micro- and nano-scales and provides researchers a nexus for multidisciplinary collaboration in high-resolution imaging. We offer state-of-the-art instrumentation and services in sample preparation, X-ray micro-CT scan, transmission electron microscopy and image analysis. One main function of the facility is to prepare samples for room temperature TEM imaging or volume SEM imaging, using conventional wet chemistry, microwave techniques and high-pressure freezing/freeze substitution. Our skilled staff will assist researchers and train them in the science and practice of electron microscopy as well.
Equipment
- FEI Tecnai Spirit BioTWIN trasmission electron microscope with LaB6 gun and Gatan Oneview camera
- Zeiss Versa 730 X-ray microscope for X-ray imaging and micro-CT scan at sub-micron resolution
- Delphi integrated scanning electron microscope and fluorescence microscope
- Wohlwend HPF Compact 03 high-pressure freezer
- Leica EM ICE high pressure freezer
- Leica EM AFS2 automatic freeze-substitution system for processing samples prepared by high-pressure freezing or progressive lowering of temperature (PLT) methods
- Built-in UV lamp for UV-catalyzed resin polymerization at low temperature
- Leica UC7 and UC6 ultramicrotomes for room temperature and cryo semi-thin and ultra-thin sectioning. Each microtome is positioned in a separate room with adequate bench space and ancillary specimen preparation equipment so as to be self-contained sectioning areas
- Pelco BioWave Pro microwave oven with ColdSpot, SteadyTemp, and vacuum chamber options for maximum microwave sample preparation flexibility
- Tousimis Autosamdri-931 critical point dryer
- Leica EM ACE600 sputter coater
- Leica VT1200S Vibratome
- Precisionary Instruments Compresstome VF-510-0Z vibratome
Services
- Sample fixation and processing
- Sample staining and embedding for TEM or volume SEM
- Ultrathin sectioning on resin-embedded samples for TEM
- Cryo sectioning on frozen hydrate samples
- TEM imaging
- Immuno EM
- Micro CT scan
- High pressure freezing for cryo light microscopy, cryo EM or freeze substitution
- Development of protocols for special research needs
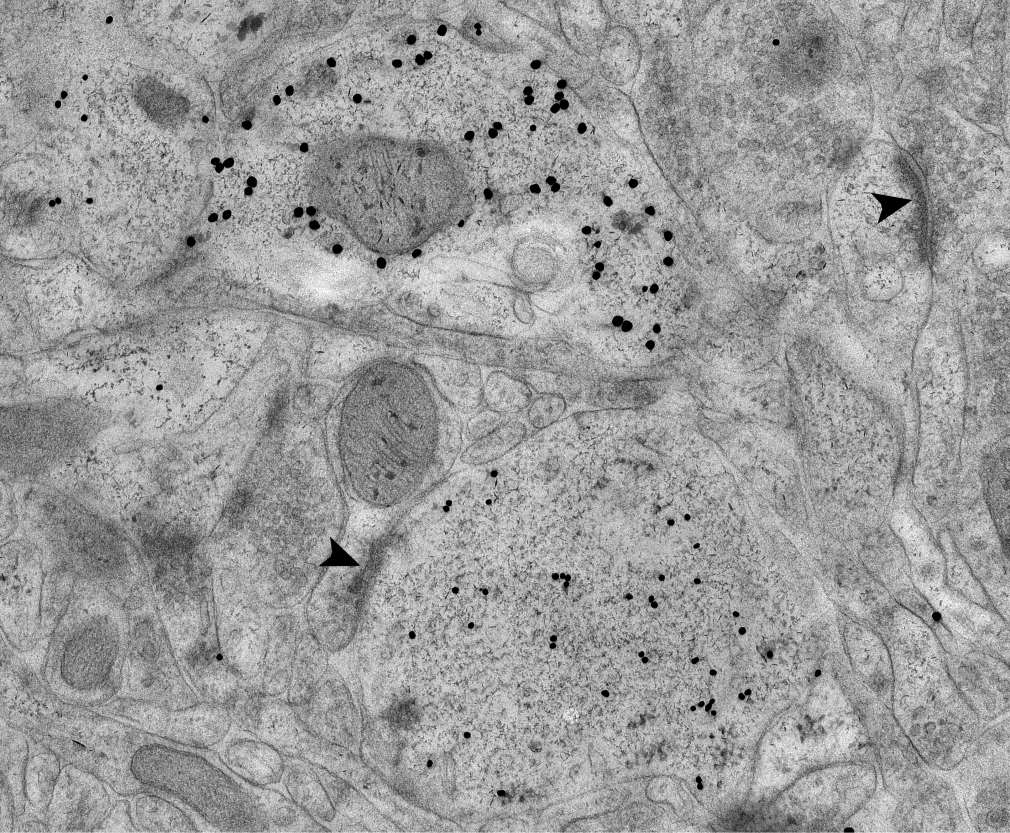
Dr. Wei-Ping Li completed her Ph.D. in cell biology with Jürgen Roth, the “immunogold master” who pioneered the use of immunogold EM on thin sections (Sedwick 2013). Following in the footsteps of her mentor, Dr. Li is Janelia’s immunogold expert, developing protocols that enable researchers to analyze connectivity between identified neurons at the EM level.
Double immunogold labeling in mouse brain is used to distinguish different cell types. Protocol was optimized to preserve antigenicity and synaptic ultrastructure (arrows).
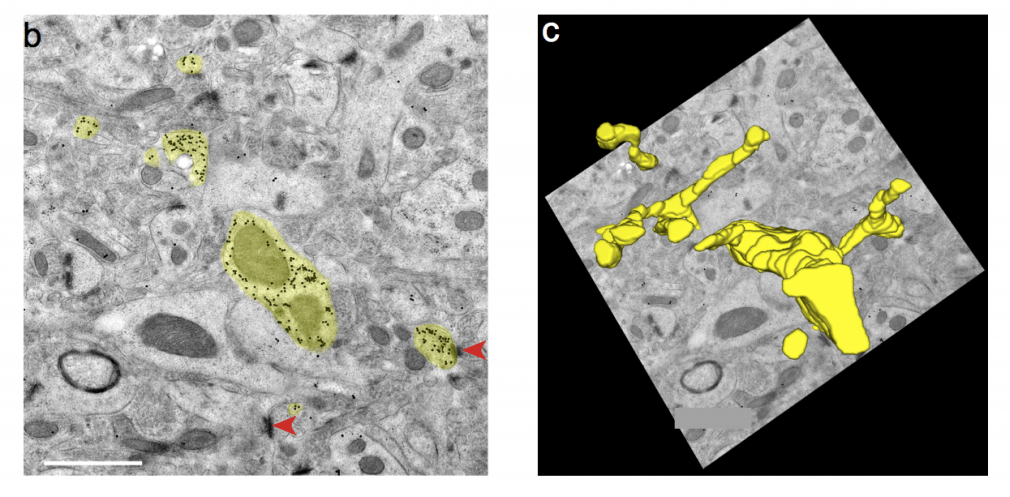
Neuron expressing a fluorescent protein engineered by the Looger Lab was reconstructed from immunogold labeled serial sections of the mouse brain. Synapses (red arrows) are readily identifiable. Figure is modified from Viswanathan et al. (2015).