Filter
Associated Lab
- Aguilera Castrejon Lab (2) Apply Aguilera Castrejon Lab filter
- Ahrens Lab (57) Apply Ahrens Lab filter
- Aso Lab (42) Apply Aso Lab filter
- Baker Lab (19) Apply Baker Lab filter
- Betzig Lab (103) Apply Betzig Lab filter
- Beyene Lab (9) Apply Beyene Lab filter
- Bock Lab (14) Apply Bock Lab filter
- Branson Lab (51) Apply Branson Lab filter
- Card Lab (37) Apply Card Lab filter
- Cardona Lab (45) Apply Cardona Lab filter
- Chklovskii Lab (10) Apply Chklovskii Lab filter
- Clapham Lab (14) Apply Clapham Lab filter
- Cui Lab (19) Apply Cui Lab filter
- Darshan Lab (8) Apply Darshan Lab filter
- Dickson Lab (32) Apply Dickson Lab filter
- Druckmann Lab (21) Apply Druckmann Lab filter
- Dudman Lab (40) Apply Dudman Lab filter
- Eddy/Rivas Lab (30) Apply Eddy/Rivas Lab filter
- Egnor Lab (4) Apply Egnor Lab filter
- Espinosa Medina Lab (16) Apply Espinosa Medina Lab filter
- Feliciano Lab (8) Apply Feliciano Lab filter
- Fetter Lab (31) Apply Fetter Lab filter
- FIB-SEM Technology (1) Apply FIB-SEM Technology filter
- Fitzgerald Lab (16) Apply Fitzgerald Lab filter
- Freeman Lab (15) Apply Freeman Lab filter
- Funke Lab (41) Apply Funke Lab filter
- Gonen Lab (59) Apply Gonen Lab filter
- Grigorieff Lab (34) Apply Grigorieff Lab filter
- Harris Lab (54) Apply Harris Lab filter
- Heberlein Lab (13) Apply Heberlein Lab filter
- Hermundstad Lab (25) Apply Hermundstad Lab filter
- Hess Lab (76) Apply Hess Lab filter
- Ilanges Lab (2) Apply Ilanges Lab filter
- Jayaraman Lab (43) Apply Jayaraman Lab filter
- Ji Lab (33) Apply Ji Lab filter
- Johnson Lab (1) Apply Johnson Lab filter
- Karpova Lab (13) Apply Karpova Lab filter
- Keleman Lab (8) Apply Keleman Lab filter
- Keller Lab (61) Apply Keller Lab filter
- Koay Lab (2) Apply Koay Lab filter
- Lavis Lab (140) Apply Lavis Lab filter
- Lee (Albert) Lab (29) Apply Lee (Albert) Lab filter
- Leonardo Lab (19) Apply Leonardo Lab filter
- Li Lab (5) Apply Li Lab filter
- Lippincott-Schwartz Lab (103) Apply Lippincott-Schwartz Lab filter
- Liu (Yin) Lab (2) Apply Liu (Yin) Lab filter
- Liu (Zhe) Lab (59) Apply Liu (Zhe) Lab filter
- Looger Lab (137) Apply Looger Lab filter
- Magee Lab (31) Apply Magee Lab filter
- Menon Lab (12) Apply Menon Lab filter
- Murphy Lab (6) Apply Murphy Lab filter
- O'Shea Lab (6) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Pachitariu Lab (37) Apply Pachitariu Lab filter
- Pastalkova Lab (5) Apply Pastalkova Lab filter
- Pavlopoulos Lab (7) Apply Pavlopoulos Lab filter
- Pedram Lab (4) Apply Pedram Lab filter
- Podgorski Lab (16) Apply Podgorski Lab filter
- Reiser Lab (46) Apply Reiser Lab filter
- Riddiford Lab (20) Apply Riddiford Lab filter
- Romani Lab (36) Apply Romani Lab filter
- Rubin Lab (109) Apply Rubin Lab filter
- Saalfeld Lab (47) Apply Saalfeld Lab filter
- Satou Lab (1) Apply Satou Lab filter
- Scheffer Lab (38) Apply Scheffer Lab filter
- Schreiter Lab (51) Apply Schreiter Lab filter
- Sgro Lab (1) Apply Sgro Lab filter
- Shroff Lab (31) Apply Shroff Lab filter
- Simpson Lab (18) Apply Simpson Lab filter
- Singer Lab (37) Apply Singer Lab filter
- Spruston Lab (58) Apply Spruston Lab filter
- Stern Lab (75) Apply Stern Lab filter
- Sternson Lab (47) Apply Sternson Lab filter
- Stringer Lab (36) Apply Stringer Lab filter
- Svoboda Lab (131) Apply Svoboda Lab filter
- Tebo Lab (11) Apply Tebo Lab filter
- Tervo Lab (9) Apply Tervo Lab filter
- Tillberg Lab (18) Apply Tillberg Lab filter
- Tjian Lab (17) Apply Tjian Lab filter
- Truman Lab (58) Apply Truman Lab filter
- Turaga Lab (41) Apply Turaga Lab filter
- Turner Lab (28) Apply Turner Lab filter
- Vale Lab (8) Apply Vale Lab filter
- Voigts Lab (3) Apply Voigts Lab filter
- Wang (Meng) Lab (23) Apply Wang (Meng) Lab filter
- Wang (Shaohe) Lab (6) Apply Wang (Shaohe) Lab filter
- Wu Lab (8) Apply Wu Lab filter
- Zlatic Lab (26) Apply Zlatic Lab filter
- Zuker Lab (5) Apply Zuker Lab filter
Associated Project Team
- CellMap (12) Apply CellMap filter
- COSEM (3) Apply COSEM filter
- FIB-SEM Technology (5) Apply FIB-SEM Technology filter
- Fly Descending Interneuron (12) Apply Fly Descending Interneuron filter
- Fly Functional Connectome (14) Apply Fly Functional Connectome filter
- Fly Olympiad (5) Apply Fly Olympiad filter
- FlyEM (56) Apply FlyEM filter
- FlyLight (50) Apply FlyLight filter
- GENIE (47) Apply GENIE filter
- Integrative Imaging (6) Apply Integrative Imaging filter
- Larval Olympiad (2) Apply Larval Olympiad filter
- MouseLight (18) Apply MouseLight filter
- NeuroSeq (1) Apply NeuroSeq filter
- ThalamoSeq (1) Apply ThalamoSeq filter
- Tool Translation Team (T3) (27) Apply Tool Translation Team (T3) filter
- Transcription Imaging (45) Apply Transcription Imaging filter
Associated Support Team
- Project Pipeline Support (5) Apply Project Pipeline Support filter
- Anatomy and Histology (18) Apply Anatomy and Histology filter
- Cryo-Electron Microscopy (40) Apply Cryo-Electron Microscopy filter
- Electron Microscopy (18) Apply Electron Microscopy filter
- Gene Targeting and Transgenics (11) Apply Gene Targeting and Transgenics filter
- High Performance Computing (7) Apply High Performance Computing filter
- Integrative Imaging (18) Apply Integrative Imaging filter
- Invertebrate Shared Resource (40) Apply Invertebrate Shared Resource filter
- Janelia Experimental Technology (37) Apply Janelia Experimental Technology filter
- Management Team (1) Apply Management Team filter
- Molecular Genomics (15) Apply Molecular Genomics filter
- Primary & iPS Cell Culture (14) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (50) Apply Project Technical Resources filter
- Quantitative Genomics (20) Apply Quantitative Genomics filter
- Scientific Computing (95) Apply Scientific Computing filter
- Viral Tools (14) Apply Viral Tools filter
- Vivarium (7) Apply Vivarium filter
Publication Date
- 2025 (199) Apply 2025 filter
- 2024 (211) Apply 2024 filter
- 2023 (158) Apply 2023 filter
- 2022 (166) Apply 2022 filter
- 2021 (175) Apply 2021 filter
- 2020 (177) Apply 2020 filter
- 2019 (177) Apply 2019 filter
- 2018 (206) Apply 2018 filter
- 2017 (186) Apply 2017 filter
- 2016 (191) Apply 2016 filter
- 2015 (195) Apply 2015 filter
- 2014 (190) Apply 2014 filter
- 2013 (136) Apply 2013 filter
- 2012 (112) Apply 2012 filter
- 2011 (98) Apply 2011 filter
- 2010 (61) Apply 2010 filter
- 2009 (56) Apply 2009 filter
- 2008 (40) Apply 2008 filter
- 2007 (21) Apply 2007 filter
- 2006 (3) Apply 2006 filter
2758 Janelia Publications
Showing 1341-1350 of 2758 resultsNitrate (NO3-) uptake and distribution are critical to plant life. Although the upstream regulation of nitrate uptake and downstream responses to nitrate in a variety of cells have been well-studied, it is still not possible to directly visualize the spatial and temporal distribution of nitrate with high resolution at the cellular level. Here, we report a nuclear-localized, genetically encoded biosensor, nlsNitraMeter3.0, for the quantitative visualization of nitrate distribution in Arabidopsis thaliana. The biosensor tracked the spatiotemporal distribution of nitrate along the primary root axis and disruptions by genetic mutation of transport (low nitrate uptake) and assimilation (high nitrate accumulation). The developed biosensor effectively monitors nitrate concentrations at cellular level in real time and spatiotemporal changes during the plant life cycle.
Much focus has shifted towards understanding how glial dysfunction contributes to age-related neurodegeneration due to the critical roles glial cells play in maintaining healthy brain function. Cell-cell interactions, which are largely mediated by cell-surface proteins, control many critical aspects of development and physiology; as such, dysregulation of glial cell-surface proteins in particular is hypothesized to play an important role in age-related neurodegeneration. However, it remains technically difficult to profile glial cell-surface proteins in intact brains. Here, we applied a cell-surface proteomic profiling method to glial cells from intact brains in Drosophila, which enabled us to fully profile cell-surface proteomes in-situ, preserving native cell-cell interactions that would otherwise be omitted using traditional proteomics methods. Applying this platform to young and old flies, we investigated how glial cell-surface proteomes change during aging. We identified candidate genes predicted to be involved in brain aging, including several associated with neural development and synapse wiring molecules not previously thought to be particularly active in glia. Through a functional genetic screen, we identified one surface protein, DIP-β, which is down-regulated in old flies and can increase fly lifespan when overexpressed in adult glial cells. We further performed whole-head single-nucleus RNA-seq, and revealed that DIP-β overexpression mainly impacts glial and fat cells. We also found that glial DIP-β overexpression was associated with improved cell-cell communication, which may contribute to the observed lifespan extension. Our study is the first to apply in-situ cell-surface proteomics to glial cells in Drosophila, and to identify DIP-β as a potential glial regulator of brain aging.Competing Interest StatementThe authors have declared no competing interest.The original mass spectra and the protein sequence databases used for searches have been deposited in the public proteomics repository MassIVE (http://massive.ucsd.edu) (username: MSV000099083; password: glial). These datasets will be made public upon acceptance of the manuscript. Original proteomic data prior to analyses is provided in the Supplementary Table 1. snRNA-seq data has been deposited to NCBI Gene Expression Omnibus (GSE308135).
Although most proteins conform to the classical one-structure/one-function paradigm, an increasing number of proteins with dual structures and functions have been discovered. In response to cellular stimuli, such proteins undergo structural changes sufficiently dramatic to remodel even their secondary structures and domain organization. This "fold-switching" capability fosters protein multi-functionality, enabling cells to establish tight control over various biochemical processes. Accurate predictions of fold-switching proteins could both suggest underlying mechanisms for uncharacterized biological processes and reveal potential drug targets. Recently, we developed a prediction method for fold-switching proteins using structure-based thermodynamic calculations and discrepancies between predicted and experimentally determined protein secondary structure. Here we seek to leverage the negative information found in these secondary structure prediction discrepancies. To do this, we quantified secondary structure prediction accuracies of 192 known fold-switching regions (FSRs) within solved protein structures found in the Protein Data Bank (PDB). We find that the secondary structure prediction accuracies for these FSRs vary widely. Inaccurate secondary structure predictions are strongly associated with fold-switching proteins compared to equally long segments of non-fold-switching proteins selected at random. These inaccurate predictions are enriched in helix-to-strand and strand-to-coil discrepancies. Finally, we find that most proteins with inaccurate secondary structure predictions are underrepresented in the PDB compared with their alternatively folded cognates, suggesting that unequal representation of fold-switching conformers within the PDB could be an important cause of inaccurate secondary structure predictions. These results demonstrate that inconsistent secondary structure predictions can serve as a useful preliminary marker of fold switching. This article is protected by copyright. All rights reserved.
The endoplasmic reticulum (ER) is an expansive, membrane-enclosed organelle that plays crucial roles in numerous cellular functions. We used emerging superresolution imaging technologies to clarify the morphology and dynamics of the peripheral ER, which contacts and modulates most other intracellular organelles. Peripheral components of the ER have classically been described as comprising both tubules and flat sheets. We show that this system consists almost exclusively of tubules at varying densities, including structures that we term ER matrices. Conventional optical imaging technologies had led to misidentification of these structures as sheets because of the dense clustering of tubular junctions and a previously uncharacterized rapid form of ER motion. The existence of ER matrices explains previous confounding evidence that had indicated the occurrence of ER “sheet” proliferation after overexpression of tubular junction–forming proteins.
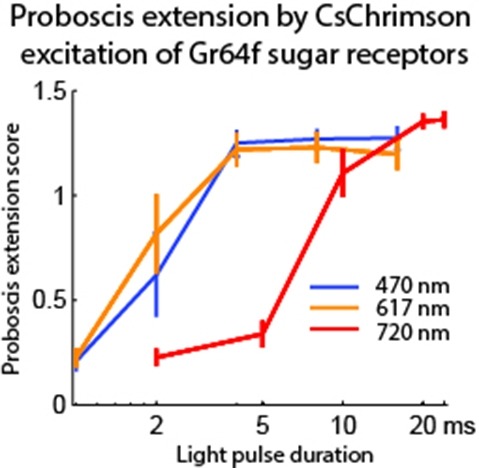
Optogenetic tools enable examination of how specific cell types contribute to brain circuit functions. A long-standing question is whether it is possible to independently activate two distinct neural populations in mammalian brain tissue. Such a capability would enable the study of how different synapses or pathways interact to encode information in the brain. Here we describe two channelrhodopsins, Chronos and Chrimson, discovered through sequencing and physiological characterization of opsins from over 100 species of alga. Chrimson’s excitation spectrum is red shifted by 45 nm relative to previous channelrhodopsins and can enable experiments in which red light is preferred. We show minimal visual system-mediated behavioral interference when using Chrimson in neurobehavioral studies in Drosophila melanogaster. Chronos has faster kinetics than previous channelrhodopsins yet is effectively more light sensitive. Together these two reagents enable two-color activation of neural spiking and downstream synaptic transmission in independent neural populations without detectable cross-talk in mouse brain slice.
SUMMARY: INFERNAL builds consensus RNA secondary structure profiles called covariance models (CMs), and uses them to search nucleic acid sequence databases for homologous RNAs, or to create new sequence- and structure-based multiple sequence alignments. AVAILABILITY: Source code, documentation and benchmark downloadable from http://infernal.janelia.org. INFERNAL is freely licensed under the GNU GPLv3 and should be portable to any POSIX-compliant operating system, including Linux and Mac OS/X.
SUMMARY: Infernal builds probabilistic profiles of the sequence and secondary structure of an RNA family called covariance models (CMs) from structurally annotated multiple sequence alignments given as input. Infernal uses CMs to search for new family members in sequence databases and to create potentially large multiple sequence alignments. Version 1.1 of Infernal introduces a new filter pipeline for RNA homology search based on accelerated profile hidden Markov model (HMM) methods and HMM-banded CM alignment methods. This enables \~{}100-fold acceleration over the previous version and \~{}10 000-fold acceleration over exhaustive non-filtered CM searches. AVAILABILITY: Source code, documentation and the benchmark are downloadable from http://infernal.janelia.org. Infernal is freely licensed under the GNU GPLv3 and should be portable to any POSIX-compliant operating system, including Linux and Mac OS/X. Documentation includes a user’s guide with a tutorial, a discussion of file formats and user options and additional details on methods implemented in the software. CONTACT: nawrockie@janelia.hhmi.org.
Live-cell imaging and particle tracking provide rich information on mechanisms of intracellular transport. However, trajectory analysis procedures to infer complex transport dynamics involving stochastic switching between active transport and diffusive motion are lacking. We applied Bayesian model selection to hidden Markov modeling to infer transient transport states from trajectories of mRNA-protein complexes in live mouse hippocampal neurons and metaphase kinetochores in dividing human cells. The software is available at http://hmm-bayes.org/.