Main Menu (Mobile)- Block
- Overview
-
Support Teams
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Primary & iPS Cell Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing Software
- Scientific Computing Systems
- Viral Tools
- Vivarium
- Open Science
- You + Janelia
- About Us
Main Menu - Block
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Primary & iPS Cell Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing Software
- Scientific Computing Systems
- Viral Tools
- Vivarium
Gonen Lab - Research
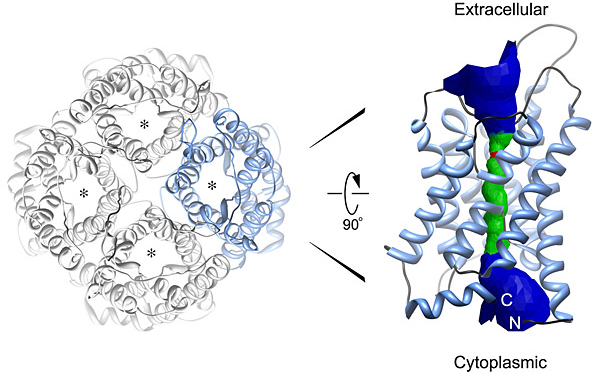
Structure of the AQP0 water channel. Left, the AQP tetramer in top view. Right, side view of a monomer. The channel is formed by a bundle of six transmembrane α-helices that pack against one another, forming a hydrophilic pore at the center.
From Andrews, S.A., Reichow, L.S., and Gonen T. 2008. IUBMB Life 60:430–436. © 2008, with permission from John Wiley & Sons, Inc.
Structure of the DMPC membrane surrounding the AQP0-mediated membrane junction. Two complete membranes (lipid bilayers) were observed surrounding the AQP0 junction. The bilayers are as indicated. Intricate lipid-protein interactions can be seen, shedding light on how lipids affect membrane protein structure and function.
Adapted from Gonen, T., Cheng, Y., Sliz, P., Hiroaki, Y., Fujiyoshi, Y., Harrison, S.C., and Walz, T. 2005.Nature 438:633–638.
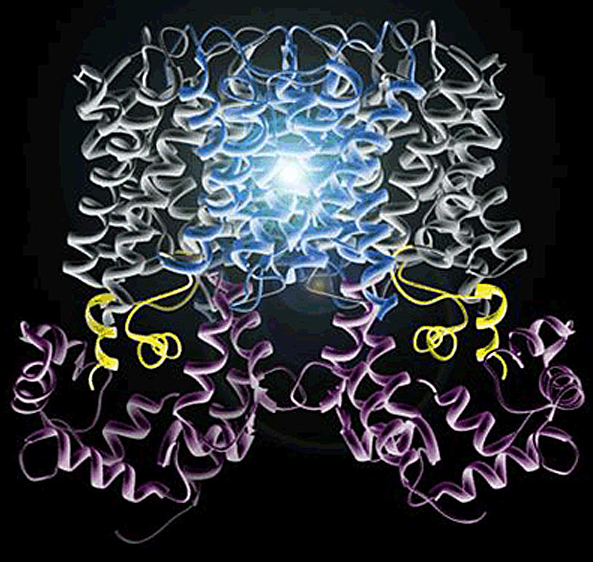
Model of the AQP0-calmodulin complex. Although the AQP0 tetramer contains four CaM-binding sites, only two CaMs bind. These CaMs appear to cap the bottom of two AQP0 monomers within the tetramer. The overall permeability for the AQP0 tetramer is halved.
Adapted from Reichow, L.S., and Gonen, T. 2008. Structure 16:1389–1398.
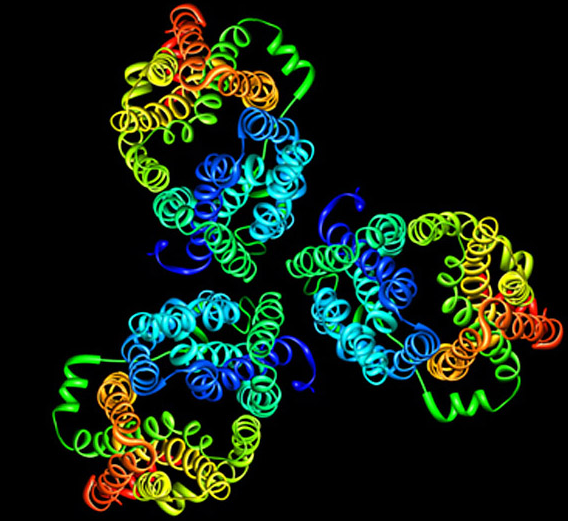
Model of the E. coli sugar transporter GalP. GalP coupled the movement of glucose or galactose to a proton. The 2D crystals indicate that the protein is a trimer, although the reason behind this is still unclear.
Image provided by Tamir Gonen. See also Zheng, H., Taraska, J., Merz, A., and Gonen, T. 2010.Journal of Molecular Biology 396:593–601.
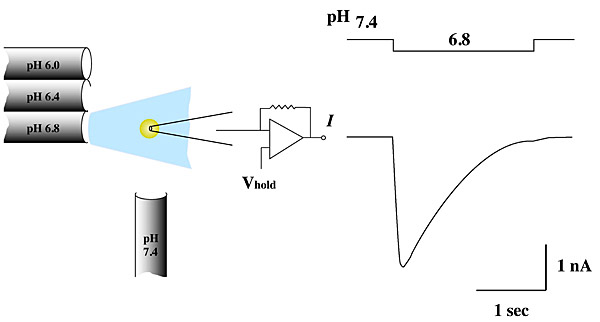
Electrophysiology and channel recordings. Patch clamping, together with other fluorescence-based methods, is used to study membrane protein function. This image depicts the activation of the acid-sensing channel ASIC in response to a change in pH.
Image provided by Ed McCleskey (HHMI).
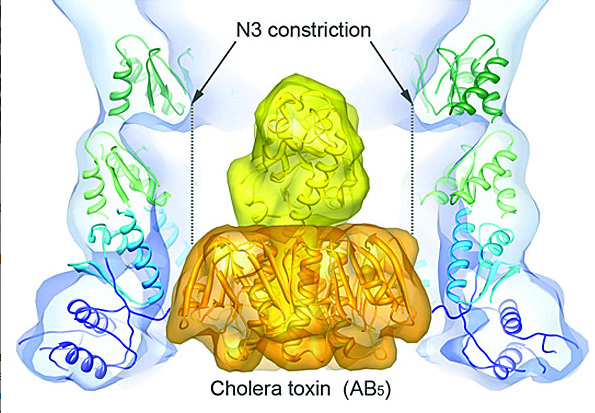
Model of the cholera toxin docked in the periplasmic vestibule of the cholera secretion channel EpsD. The type II secretion system (T2SS) is a macromolecular complex spanning the inner and outer membranes of Gram-negative bacteria. Remarkably, the T2SS secretes folded proteins, including multimeric assemblies such as cholera toxin.
Adapted from Reichow, S.L., Korotkov, K.V., Hol, W.G.J., and Gonen, T. 2010. Nature Structural & Molecular Biology 17:1226–1232.
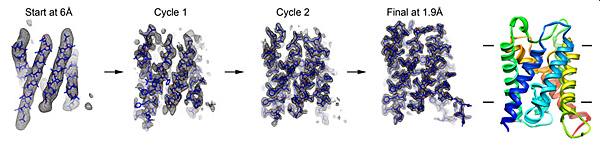
Fragment-based phase extension. The Gonen lab developed a new method for determining membrane protein structure by electron diffraction. The image depicts the stepwise structural refinement of the water channel AQP0, starting with the 6Å experimental map as it was extended to 1.9Å experimental diffraction data.
From Wisedchaisri, G., and Gonen, T. 2011. Structure 19:976–987. © 2011, with permission from Elsevier.