Filter
Associated Lab
- Ahrens Lab (2) Apply Ahrens Lab filter
- Aso Lab (1) Apply Aso Lab filter
- Baker Lab (1) Apply Baker Lab filter
- Branson Lab (1) Apply Branson Lab filter
- Druckmann Lab (3) Apply Druckmann Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Hermundstad Lab (9) Apply Hermundstad Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Remove Jayaraman Lab filter Jayaraman Lab
- Ji Lab (1) Apply Ji Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Looger Lab (10) Apply Looger Lab filter
- Podgorski Lab (1) Apply Podgorski Lab filter
- Reiser Lab (2) Apply Reiser Lab filter
- Romani Lab (5) Apply Romani Lab filter
- Rubin Lab (6) Apply Rubin Lab filter
- Saalfeld Lab (1) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Schreiter Lab (9) Apply Schreiter Lab filter
- Svoboda Lab (9) Apply Svoboda Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2025 (1) Apply 2025 filter
- 2024 (3) Apply 2024 filter
- 2023 (1) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (1) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (3) Apply 2018 filter
- 2017 (4) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (4) Apply 2015 filter
- 2014 (1) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (2) Apply 2012 filter
- 2011 (2) Apply 2011 filter
- 2010 (2) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2007 (1) Apply 2007 filter
- 2006 (1) Apply 2006 filter
- 2003 (1) Apply 2003 filter
Type of Publication
46 Publications
Showing 11-20 of 46 resultsThe neural circuits responsible for animal behavior remain largely unknown. We summarize new methods and present the circuitry of a large fraction of the brain of the fruit fly . Improved methods include new procedures to prepare, image, align, segment, find synapses in, and proofread such large data sets. We define cell types, refine computational compartments, and provide an exhaustive atlas of cell examples and types, many of them novel. We provide detailed circuits consisting of neurons and their chemical synapses for most of the central brain. We make the data public and simplify access, reducing the effort needed to answer circuit questions, and provide procedures linking the neurons defined by our analysis with genetic reagents. Biologically, we examine distributions of connection strengths, neural motifs on different scales, electrical consequences of compartmentalization, and evidence that maximizing packing density is an important criterion in the evolution of the fly's brain.
Many animals use an internal sense of direction to guide their movements through the world. Neurons selective to head direction are thought to support this directional sense and have been found in a diverse range of species, from insects to primates, highlighting their evolutionary importance. Across species, most head-direction networks share four key properties: a unique representation of direction at all times, persistent activity in the absence of movement, integration of angular velocity to update the representation, and the use of directional cues to correct drift. The dynamics of theorized network structures called ring attractors elegantly account for these properties, but their relationship to brain circuits is unclear. Here, we review experiments in rodents and flies that offer insights into potential neural implementations of ring attractor networks. We suggest that a theory-guided search across model systems for biological mechanisms that enable such dynamics would uncover general principles underlying head-direction circuit function. Expected final online publication date for the , Volume 43 is July 8, 2020. Please see http://www.annualreviews.org/page/journal/pubdates for revised estimates.
Here we design and optimize a genetically encoded fluorescent indicator, iAChSnFR, for the ubiquitous neurotransmitter acetylcholine, based on a bacterial periplasmic binding protein. iAChSnFR shows large fluorescence changes, rapid rise and decay kinetics, and insensitivity to most cholinergic drugs. iAChSnFR revealed large transients in a variety of slice and in vivo preparations in mouse, fish, fly and worm. iAChSnFR will be useful for the study of acetylcholine in all animals.
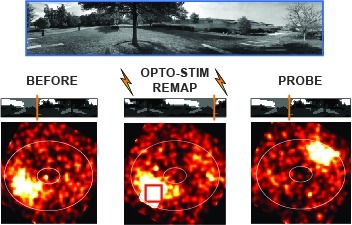
Many animals rely on an internal heading representation when navigating in varied environments. How this representation is linked to the sensory cues that define different surroundings is unclear. In the fly brain, heading is represented by 'compass' neurons that innervate a ring-shaped structure known as the ellipsoid body. Each compass neuron receives inputs from 'ring' neurons that are selective for particular visual features; this combination provides an ideal substrate for the extraction of directional information from a visual scene. Here we combine two-photon calcium imaging and optogenetics in tethered flying flies with circuit modelling, and show how the correlated activity of compass and visual neurons drives plasticity, which flexibly transforms two-dimensional visual cues into a stable heading representation. We also describe how this plasticity enables the fly to convert a partial heading representation, established from orienting within part of a novel setting, into a complete heading representation. Our results provide mechanistic insight into the memory-related computations that are essential for flexible navigation in varied surroundings.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The resulting jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and may allow tracking larger populations of neurons using two-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
Clock neurons generate circadian rhythms in behavioral activity, but the relevant pathways remain poorly understood. In this issue of Neuron, Liang et al. (2019) show that distinct clock neurons independently drive movement-promoting “ring neurons” in Drosophila through dopaminergic relays to support morning and evening locomotor activity.
View Publication PageStudying the intertwined roles of sensation, experience, and directed action in navigation has been facilitated by the development of virtual reality (VR) environments for head-fixed animals, allowing for quantitative measurements of behavior in well-controlled conditions. VR has long featured in studies of Drosophila melanogaster, but these experiments have typically allowed the fly to change only its heading in a visual scene and not its position. Here we explore how flies move in two dimensions (2D) using a visual VR environment that more closely captures an animal's experience during free behavior. We show that flies' 2D interaction with landmarks cannot be automatically derived from their orienting behavior under simpler one-dimensional (1D) conditions. Using novel paradigms, we then demonstrate that flies in 2D VR adapt their behavior in response to optogenetically delivered appetitive and aversive stimuli. Much like free-walking flies after encounters with food, head-fixed flies exploring a 2D VR respond to optogenetic activation of sugar-sensing neurons by initiating a local search, which appears not to rely on visual landmarks. Visual landmarks can, however, help flies to avoid areas in VR where they experience an aversive, optogenetically generated heat stimulus. By coupling aversive virtual heat to the flies' presence near visual landmarks of specific shapes, we elicit selective learned avoidance of those landmarks. Thus, we demonstrate that head-fixed flies adaptively navigate in 2D virtual environments, but their reliance on visual landmarks is context dependent. These behavioral paradigms set the stage for interrogation of the fly brain circuitry underlying flexible navigation in complex multisensory environments.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics, and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and tracking large populations of neurons using 2-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
Seizures induced by visual stimulation (photosensitive epilepsy; PSE) represent a common type of epilepsy in humans, but the molecular mechanisms and genetic drivers underlying PSE remain unknown, and no good genetic animal models have been identified as yet. Here, we show an animal model of PSE, in , owing to defective cortex glia. The cortex glial membranes are severely compromised in ceramide phosphoethanolamine synthase ()-null mutants and fail to encapsulate the neuronal cell bodies in the neuronal cortex. Expression of human sphingomyelin synthase 1, which synthesizes the closely related ceramide phosphocholine (sphingomyelin), rescues the cortex glial abnormalities and PSE, underscoring the evolutionarily conserved role of these lipids in glial membranes. Further, we show the compromise in plasma membrane structure that underlies the glial cell membrane collapse in mutants and leads to the PSE phenotype.
The central complex is a highly conserved insect brain region composed of morphologically stereotyped neurons that arborize in distinctively shaped substructures. The region is implicated in a wide range of behaviors and several modeling studies have explored its circuit computations. Most studies have relied on assumptions about connectivity between neurons based on their overlap in light microscopy images. Here, we present an extensive functional connectome of Drosophila melanogaster's central complex at cell-type resolution. Using simultaneous optogenetic stimulation, calcium imaging and pharmacology, we tested the connectivity between 70 presynaptic-to-postsynaptic cell-type pairs. We identi1ed numerous inputs to the central complex, but only a small number of output channels. Additionally, the connectivity of this highly recurrent circuit appears to be sparser than anticipated from light microscopy images. Finally, the connectivity matrix highlights the potentially critical role of a class of bottleneck interneurons. All data is provided for interactive exploration on a website.