The Unique Capabilities of acMFM
The aberration-corrected multifocal microscope (acMFM) system was developed by the late Dr. Mats Gustafsson at HHMI Janelia. This unique microscope is capable of rapidly imaging nine focal planes simultaneously (up to 30 z-stacks per second), and is thus perfectly suited to image rapid processes in three dimensions in living samples. This technique can be applied to conventional imaging as well as 3D single particle tracking experiments – for example, this technology has been used for 3D tracking of vesicular transport, RNA Pol II nuclear mobility, or rapid cytoskeletal rearrangement in three dimension. At Janelia, we have even successfully utilized the acMFM to follow C. elegans neuronal activity.
Movie 1: acMFM applied to tracking of single Sox2 transcription factors
within the cell nucleus. Information within a 4-micron z-depth was
captured simultaneously without moving the objective or sample. Taken
from Chen et al., Cell 156(6), 1274–1285 (2014)
Movie 2: Raw video, showing 9 simultaneous focal planes of the unc-47
GABAergic motor neurons of this C. elegans embryo expressing a green
fluorescent protein. Taken from Abrahamsson, S. et al. Nat. Methods 10,
60–3 (2013).
Basic Principles
Most current technologies for taking three-dimensional images rely on serially scanning the sample and/or objective in the axial (Z) dimension to build up a 3D image. Although these various methods have improved significantly, they can still result in ambiguity as the 3D image is not collected instantaneously. Additionally, moving the sample/objective for each slice of the image stack can cause mechanical perturbations in the sample, leading to further difficulties in interpreting the information from the 3D image.
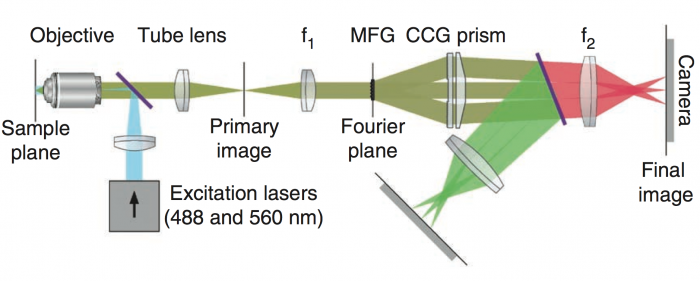
Figure 1. General microscope schematic. Adapted from Abrahamsson, et al. Nat Meth. 2013
The acMFM at Janelia's AIC consists of an epi-illumination setup that collects nine aberration-corrected focal planes simultaneously via a custom-made multifocal diffraction grating (MFG) (Figure 1). In this way, a 3D image is collected without moving the sample or any part of the microscpe. Aberration correction within the MFG removes spherical aberrations that occur in commercial MFMs as a result of shifting the image away from the nominal focal plane. The system also corrects for chromatic aberrations that occur from the MFG by using a separate chromatic correction grating (CCG) and prism (Figure 2). The entire acMFM system is designed to interface with the camera port of a commercial high-resolution epi-fluorescent microscope, and is thus relatively simple to operate. The system maintains high sensitivity and lowers photodamage due to reduced total exposure times. It has been used to image weakly fluorescent samples (including single fluorophores). The separation between focal planes depths (ΔZ) is determined by the multifocal grating. With one of our gratings (ΔZ ~400nm) one achieves ca. 3µm of depth information per z-stack, with full Nyquist sampling rate. Each full z-stack can be acquired at a rate of of to 30Hz - permitting the capture of very rapid biological processes. The current system configuration allows for simultaneous two color imaging with each color being collected by a separate camera (shown in Figure 1 as red and green).
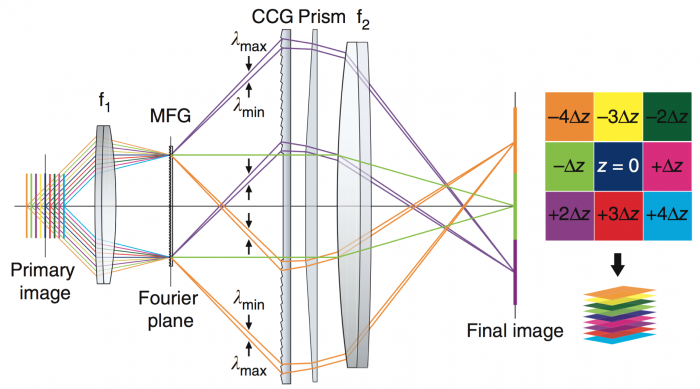
Figure 2. Multiple focal planes focused on CCD. Adapted from Abrahamsson, et al. Nat Meth. 2013
acMFM Strengths
• Simultaneous capture of image z-stacks comprised of 9 separate, aberration- and chromatic-corrected focal planes
• Perfect temporal alignment across each z-stack eliminates ambiguity inherent to sequential z-sectioning
• Rapid acquisition of z-stacks, up to 35 stacks per second
• Simultaneous two color imaging (green and red channel)
acMFM Limitations
• The field of view in multi-focal mode is limited to ca. 35x35 microns (60x magnification) or ca. 20x20 microns (100x magnification). Larger field of view is available in single focal plane mode.
• Sample is illuminated in epi-fluorescent mode (i.e. illuminated along Z axis), which can cause issues with out-of-focus fluorescence beyond desired 2D stack depth.
• Epi-fluorescent illumination may require optimization to further reduce fluorophore photobleaching or phototoxicity.
• Ability to distinguish 9 focal planes relies on the multifocal grating, which causes a 10-20% reduction in detection efficiency compared to typical widefield fluorescent techniques.
• Spatial resolution is diffraction limited, and dependent on microscope configuration (i.e. objective and excitation/emission wavelength)
Lasers
• 405 nm
• 488 nm
• 561 nm
• 635 nm
Dichroics (used with LED white light source, single wavelength illumination)
• DAPI-1160B (Semrock)
• CFP-2432C (Semrock)
• FITC-3540C (Semrock)
• TRITC-B (Semrock)
• Cy5-4040C (Semrock)
Dichroic (used with laser excitation, multi wavelength illumination)
• Di01-R405/488/561/635 (Semrock)
Filters
• FF01-525/45 (Semrock)
• FF01-593/40 (Semrock)
• FF01-676/37 (Semrock)
Objectives
• 100x NA 1.45 Oil (Nikon)
• 60x NA 1.4 Oil (Nikon)
• 60x NA 1.2 Water (Nikon)
• 20x NA 0.75 Air (Nikon)
Camera
• iXon3-DU897E EMCCD (Andor Technologies)
Current High Efficiency Multifocal Gratings
| Grating | Objective/NA | Emission Wavelength (nm)* | Z-separation (nm) |
| 1 | 60xwater / 1.20 | 515 | 400 |
| 2 | 60x water / 1.20 | 600 | 400 |
| 3 | 60x oil / 1.40 | 515 | 400 |
| 4 | 60x oil / 1.40 | 600 | 400 |
| 5 | 100x oil / 1.45 | 515 | 400 |
| 6 | 100x oil / 1.45 | 600 | 400 |
Suggested Reading
1. Abrahamsson, S. et al. Fast multicolor 3D imaging using aberration-corrected multifocus microscopy. Nat Methods 10, 60–63 (2013).
2. Chen, J. et al. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 156, 1274–1285 (2014).
Example Data
