A T Cell (Orange) approaching an antigen presenting cell (APC, Blue). The left two panels show two orientations of the cells coming into contact and forming a mature immunological synapse. The right two panels show the same two viewpoints of the T Cell (APC made invisible) during this interaction. Images were acquired every 1.3 sec over 430 time points.
Unique System Features
- High speed imaging for live samples with frame rates near 300 slices per second
- Significant reduction of photobleaching and phototoxicity due to use of focal-plane illumination
- Excellent optical sectioning capability (sheet thickness typically <600 nm)
- In ideal samples, capable of 230 x 230 x 370 nm X-Y-Z resolution
- Volumetric field of view approximately 50 x 50 x 50 µm X-Y-Z
- Capable of imaging approximately 50 µm deep in large specimens, e.g. Drosophila or zebrafish embryos
- Two sCMOS cameras for FRET imaging
- Continuous perfusion of pre-warmed media available
One of the biggest challenges in imaging live processes is observing cellular and molecular behavior in context, e.g. how cells and macromolecular networks work in their native microenvironment. The technical hurdle in achieving this goal has always been the simultaneous pursuit of imaging depth, speed, resolution and specimen viability. The development of light sheet microscopy, which couples a separate excitation lens in perpendicular axis to the detection lens, has made it possible to confine the excitation of the specimen only to the volume being observed, thus greatly reducing phototoxicity, and simultaneously increasing image acquisition speed through its widefield light collection mode. [1,2,3] However, this approach is often hampered by light diffraction resulted from the Gaussian beams used in the system, which imposes limits on the optical resolution that can be physically attained.
The original implementation of a non-diffracting Bessel beam in light sheet microscopy solved this problem by delivering a virtual light sheet of submicron thickness. [4] The lattice light sheet imaging system currently housed at the AIC further improves upon this first generation configuration. By introducing a massively parallel linear array of coherently interfering Bessel beams, the microscope can generate a true light sheet, which significantly reduces data acquisition time. [7] One additional feature that is of great practical importance for biologists is the remarkably low photodamage caused by the lattice light sheet microscope. Tens of thousands of images have been taken from many specimens using this instrument without the sort of adverse effects that can be observed orders of magnitude sooner with other live cell imaging systems. The AIC lattice light sheet microscope is a powerful tool for gaining unprecedented mechanistic insights into biological processes that occur even on very fast time scales. This instrument is tailored for fast 3D imaging of molecules, cells, and embryos with high SNR and low phototoxicity. Prior to submitting a proposal to use the lattice light sheet microscope, please read two blog articles we have prepared on sample preparation and understanding objective-sample orientation. It is strongly encouraged that you read these articles when considering how your project might work on the lattice light sheet microscope.
The distribution of C. Elegans embryonic mid-body associated protein (AIR-2, green) relative to the plasma membrane and histone 2B (red). Images were acquired every 60 sec starting at the 2 cell stage and continuing through development.
System Specifications
Currently Available Lasers (in nm):
- 405 (Oxxius diode laser, rated 100 mW)
- 445 (Oxxius diode laser, rated 100 mW)
- 488 (MPB fiber laser, rated 500 mW)
- 532 (MPB fiber laser, rated 500 mW)
- 560 (MPB fiber laser, rated 500 mW)
- 589 (MPB fiber laser, rated 500 mW)
- 642 (MPB fiber laser, rated 500 mW)
Objectives:
- Excitation: Special Optics 0.65 NA, 3.74 mm working water dipping lens
- Detection: Nikon CFI Apo LWD 25x– Water dipping, 63x magnification, 1.1 NA, 3 mm working distance, with a correction collar for adjusting to (reasonable) refractive index mismatches
Cameras
- 2x Hamamatsu Orca Flash 4.0 sCMOS
Currently available Semrock filters:
- FF02-435/40
- FF01-466/40
- FF03-525/50
- FF01-562/40
- FF01-593/46
- FF02-617/73
- FF01-629/53
- FF01-465/537/623
- FF01-446/523/600/677
- BLP01-442R
- BLP01-532R
- BLP01-488R
- BLP02-561R
- BLP01-594R
- BLP01-647R
- NOTE: Obtaining new filters for specific applications is straightforward and can be done in special cases.
Top and side volume renderings of HeLa cell filopodia expressing mEmerald-Lifeact comparing lattice light sheet mode (images acquired every 1.5 sec) and super resolution SIM mode (images acquired every 7.5 sec).
Limitations:
Although an excellent tool for biomedical imaging, the lattice light sheet system does have its limitations.
- Better resolution can be achieved through 3D-SIM or iPALM
- Unless the sample contains fluorophores that excite at the same wavelength and emit at two distinct wavelengths, all mutli-color imaging is done sequentially (per slice).
- Although capable of imaging fixed samples, designed primarily for live
- Two-photon imaging capability not available on AIC system
- Generally unable to image > 50 µm below sample surface due to optical aberrations and scattering of the sample
- Due to objective placement (detection is orthogonal to excitation) sample mounting is atypical for biological samples and requires free working space above sample (i.e. no cover slip on top). Please refer to this article for more details.
- Not optimally configured for FRAP or photoactivation experiments. Please contact AIC staff to discuss in more detail.
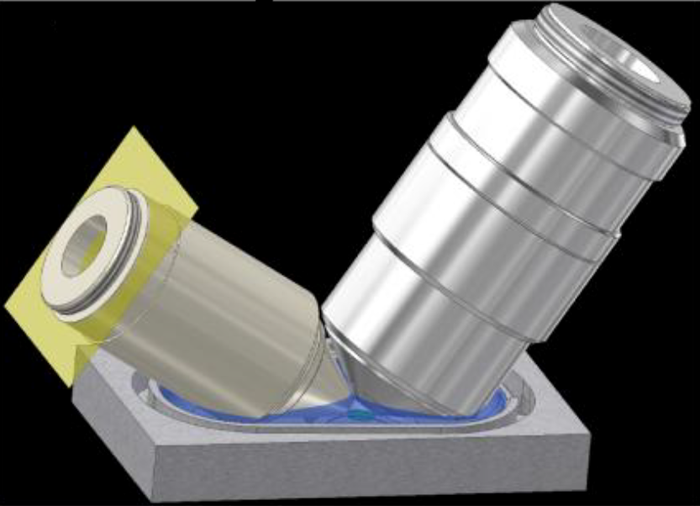
Schematic diagram of excitation objection (left) and detection objective (right) when submerged into media bath.
Suggested Reading
- Voie, A.H., Burns, D.H., & Spelman, F.A. Orthogonal-plane fluorescent optical sectioning: three-dimensional imaging of macroscopic biological specimens. J. Microsc. 170, 229-36 (1993).
- Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J., & Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plan illumination microscopy. Science 13, 1007-9 (2004).
- Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M., & Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods 10, 413-20 (2013).
- Planchon, T. A., et al. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nat. Methods 8, 417–23 (2011).
- Gao, L., Shao, L., Chen, B.C. & Betzig, E. 3D live fluorescence imaging of cellular dynamics using Bessel beam plane illumination microscopy. Nat. Protoc. 9, 1083–101 (2014).
- Gao, L., et al. Noninvasive Imaging beyond the Diffraction Limit of 3D Dynamics in Thickly Fluorescent Specimens. Cell 151, 1370–1385 (2012).
- Chen, B.C., et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, (2014).
