Glucose Sensor
Single-Fluorescent Glucose Protein Sensors
Download our Reagents Catalog here.
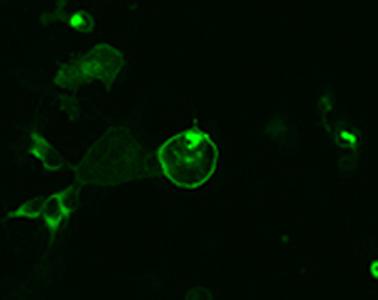
Inventors at Howard Hughes Medical Center's Janelia Research Campus have developed a strategy for creating genetically encoded, intensity-based fluorescent sensors and an associated unique set of analytical tools that can measure the presence of a single molecular analyte in a complex mixture using standard optical technology. A series of Glucose Sensors (iGlucoSnFrs) made via this technique is also available. The binding of a targeted analyte to the permuted fluorescent protein induces a dramatic conformational change in the reporter molecule and a change in the molecule's fluorescent protein properties, which is detectable using generic fluorescence detector instrumentation.
Multiple sensors of the periplasmic binding protein superfamily are available, including maltotriose and starches from a thermophilic maltotriose-binding protein (https://www.addgene.org/browse/article/4927/), glutamate from an E. coli glutamate-binding protein (https://www.addgene.org/browse/article/6319/), and phosphonates from an E. coli phosphonate-binding protein.
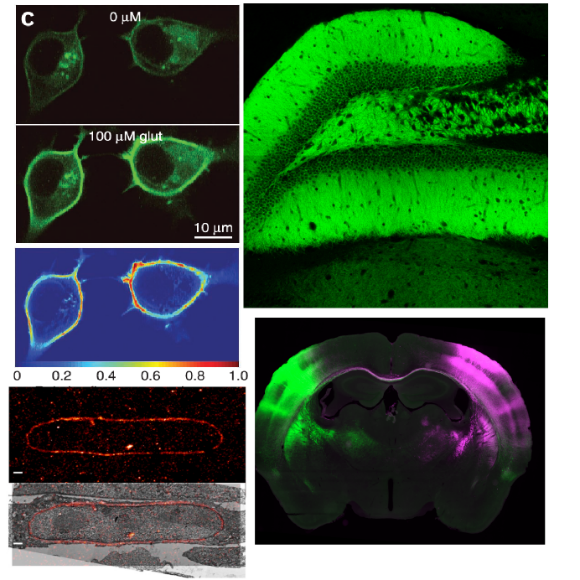
The approach also provides a unique strategy for developing a glucose sensor. Binding of glucose to its permutated fluorescent sensor protein results in a 2.5-fold increase in fluorescence signal at an emissions level of 565 nm, which allows for high-throughput screening to optimize linkers and qualitative structural analysis of local conformational changes that occur upon ligand binding, with excellent signal-to-noise ratio. The signal is easily detectable using simple instrumentation. This strategy could be beneficial for developing a continuous, reagent-free glucose sensor for diabetics and glucose sensing in model organisms, especially when monitoring organisms following strokes, brain trauma, or spinal cord injury. As well as neurodegenerative disorders such as glaucoma, Alzheimer’s and Parkinson’s disease.
The iGlucoSnFrs are GCaMP-like green sensors. They can be expressed either in the cytosol, on the plasma membrane facing out, or secreted and are available either in green only or with mRuby2 attached to the C terminus and with CMV promoters GFAP promoters, and Synapsin promoters. Please direct your inquiries to innovation@janelia.hhmi.org.
Advantages:
- Single wavelength glucose sensor is completely genetically encoded
- Significant fluorescence increases above background upon binding of glucose substrate
- Blood glucose sensor affinity is within the physiological range, and affinity variants are available
- Tunable binding site affinity and specificity under control of various promoters
- Detection using standard optical and nano-technologies
- A diverse protein super-family allows for the detection of diseases that affect glucose production.
- Allows for great detection for both
- Reports direct and accurate synaptic release
Applications:
- Blood glucose monitoring for diabetes
- In vivo imaging of extracellular and intracellular glucose
- Create in vitro and in vivo models using cell lines and animals
- The diversity of the established periplasmic binding protein superfamily sensors affords additional applications in agriculture, neurobiology, and biotechnology
Opportunity:
Available for free non-profit use and commercial licensing. Please contact innovation@janelia.hhmi.org and reference "Glucose Sensors."
For inquiries, please reference:
Janelia 2010-002