Filter
Associated Lab
- Aguilera Castrejon Lab (2) Apply Aguilera Castrejon Lab filter
- Ahrens Lab (57) Apply Ahrens Lab filter
- Aso Lab (42) Apply Aso Lab filter
- Baker Lab (19) Apply Baker Lab filter
- Betzig Lab (103) Apply Betzig Lab filter
- Beyene Lab (9) Apply Beyene Lab filter
- Bock Lab (14) Apply Bock Lab filter
- Branson Lab (51) Apply Branson Lab filter
- Card Lab (37) Apply Card Lab filter
- Cardona Lab (45) Apply Cardona Lab filter
- Chklovskii Lab (10) Apply Chklovskii Lab filter
- Clapham Lab (14) Apply Clapham Lab filter
- Cui Lab (19) Apply Cui Lab filter
- Darshan Lab (8) Apply Darshan Lab filter
- Dickson Lab (32) Apply Dickson Lab filter
- Druckmann Lab (21) Apply Druckmann Lab filter
- Dudman Lab (40) Apply Dudman Lab filter
- Eddy/Rivas Lab (30) Apply Eddy/Rivas Lab filter
- Egnor Lab (4) Apply Egnor Lab filter
- Espinosa Medina Lab (16) Apply Espinosa Medina Lab filter
- Feliciano Lab (8) Apply Feliciano Lab filter
- Fetter Lab (31) Apply Fetter Lab filter
- FIB-SEM Technology (1) Apply FIB-SEM Technology filter
- Fitzgerald Lab (16) Apply Fitzgerald Lab filter
- Freeman Lab (15) Apply Freeman Lab filter
- Funke Lab (41) Apply Funke Lab filter
- Gonen Lab (59) Apply Gonen Lab filter
- Grigorieff Lab (34) Apply Grigorieff Lab filter
- Harris Lab (54) Apply Harris Lab filter
- Heberlein Lab (13) Apply Heberlein Lab filter
- Hermundstad Lab (25) Apply Hermundstad Lab filter
- Hess Lab (76) Apply Hess Lab filter
- Ilanges Lab (2) Apply Ilanges Lab filter
- Jayaraman Lab (43) Apply Jayaraman Lab filter
- Ji Lab (33) Apply Ji Lab filter
- Johnson Lab (1) Apply Johnson Lab filter
- Karpova Lab (13) Apply Karpova Lab filter
- Keleman Lab (8) Apply Keleman Lab filter
- Keller Lab (61) Apply Keller Lab filter
- Koay Lab (2) Apply Koay Lab filter
- Lavis Lab (140) Apply Lavis Lab filter
- Lee (Albert) Lab (29) Apply Lee (Albert) Lab filter
- Leonardo Lab (19) Apply Leonardo Lab filter
- Li Lab (5) Apply Li Lab filter
- Lippincott-Schwartz Lab (102) Apply Lippincott-Schwartz Lab filter
- Liu (Yin) Lab (2) Apply Liu (Yin) Lab filter
- Liu (Zhe) Lab (59) Apply Liu (Zhe) Lab filter
- Looger Lab (137) Apply Looger Lab filter
- Magee Lab (31) Apply Magee Lab filter
- Menon Lab (12) Apply Menon Lab filter
- Murphy Lab (6) Apply Murphy Lab filter
- O'Shea Lab (6) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Pachitariu Lab (37) Apply Pachitariu Lab filter
- Pastalkova Lab (5) Apply Pastalkova Lab filter
- Pavlopoulos Lab (7) Apply Pavlopoulos Lab filter
- Pedram Lab (4) Apply Pedram Lab filter
- Podgorski Lab (16) Apply Podgorski Lab filter
- Reiser Lab (46) Apply Reiser Lab filter
- Riddiford Lab (20) Apply Riddiford Lab filter
- Romani Lab (36) Apply Romani Lab filter
- Rubin Lab (108) Apply Rubin Lab filter
- Saalfeld Lab (47) Apply Saalfeld Lab filter
- Satou Lab (1) Apply Satou Lab filter
- Scheffer Lab (38) Apply Scheffer Lab filter
- Schreiter Lab (51) Apply Schreiter Lab filter
- Sgro Lab (1) Apply Sgro Lab filter
- Shroff Lab (31) Apply Shroff Lab filter
- Simpson Lab (18) Apply Simpson Lab filter
- Singer Lab (37) Apply Singer Lab filter
- Spruston Lab (58) Apply Spruston Lab filter
- Stern Lab (75) Apply Stern Lab filter
- Sternson Lab (47) Apply Sternson Lab filter
- Stringer Lab (36) Apply Stringer Lab filter
- Svoboda Lab (131) Apply Svoboda Lab filter
- Tebo Lab (10) Apply Tebo Lab filter
- Tervo Lab (9) Apply Tervo Lab filter
- Tillberg Lab (18) Apply Tillberg Lab filter
- Tjian Lab (17) Apply Tjian Lab filter
- Truman Lab (58) Apply Truman Lab filter
- Turaga Lab (40) Apply Turaga Lab filter
- Turner Lab (28) Apply Turner Lab filter
- Vale Lab (8) Apply Vale Lab filter
- Voigts Lab (3) Apply Voigts Lab filter
- Wang (Meng) Lab (23) Apply Wang (Meng) Lab filter
- Wang (Shaohe) Lab (6) Apply Wang (Shaohe) Lab filter
- Wu Lab (8) Apply Wu Lab filter
- Zlatic Lab (26) Apply Zlatic Lab filter
- Zuker Lab (5) Apply Zuker Lab filter
Associated Project Team
- CellMap (12) Apply CellMap filter
- COSEM (3) Apply COSEM filter
- FIB-SEM Technology (5) Apply FIB-SEM Technology filter
- Fly Descending Interneuron (12) Apply Fly Descending Interneuron filter
- Fly Functional Connectome (14) Apply Fly Functional Connectome filter
- Fly Olympiad (5) Apply Fly Olympiad filter
- FlyEM (56) Apply FlyEM filter
- FlyLight (50) Apply FlyLight filter
- GENIE (47) Apply GENIE filter
- Integrative Imaging (6) Apply Integrative Imaging filter
- Larval Olympiad (2) Apply Larval Olympiad filter
- MouseLight (18) Apply MouseLight filter
- NeuroSeq (1) Apply NeuroSeq filter
- ThalamoSeq (1) Apply ThalamoSeq filter
- Tool Translation Team (T3) (27) Apply Tool Translation Team (T3) filter
- Transcription Imaging (45) Apply Transcription Imaging filter
Associated Support Team
- Project Pipeline Support (5) Apply Project Pipeline Support filter
- Anatomy and Histology (18) Apply Anatomy and Histology filter
- Cryo-Electron Microscopy (40) Apply Cryo-Electron Microscopy filter
- Electron Microscopy (18) Apply Electron Microscopy filter
- Gene Targeting and Transgenics (11) Apply Gene Targeting and Transgenics filter
- High Performance Computing (7) Apply High Performance Computing filter
- Integrative Imaging (18) Apply Integrative Imaging filter
- Invertebrate Shared Resource (40) Apply Invertebrate Shared Resource filter
- Janelia Experimental Technology (37) Apply Janelia Experimental Technology filter
- Management Team (1) Apply Management Team filter
- Molecular Genomics (15) Apply Molecular Genomics filter
- Primary & iPS Cell Culture (14) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (50) Apply Project Technical Resources filter
- Quantitative Genomics (19) Apply Quantitative Genomics filter
- Scientific Computing (95) Apply Scientific Computing filter
- Viral Tools (14) Apply Viral Tools filter
- Vivarium (7) Apply Vivarium filter
Publication Date
- 2025 (196) Apply 2025 filter
- 2024 (211) Apply 2024 filter
- 2023 (158) Apply 2023 filter
- 2022 (166) Apply 2022 filter
- 2021 (175) Apply 2021 filter
- 2020 (177) Apply 2020 filter
- 2019 (177) Apply 2019 filter
- 2018 (206) Apply 2018 filter
- 2017 (186) Apply 2017 filter
- 2016 (191) Apply 2016 filter
- 2015 (195) Apply 2015 filter
- 2014 (190) Apply 2014 filter
- 2013 (136) Apply 2013 filter
- 2012 (112) Apply 2012 filter
- 2011 (98) Apply 2011 filter
- 2010 (61) Apply 2010 filter
- 2009 (56) Apply 2009 filter
- 2008 (40) Apply 2008 filter
- 2007 (21) Apply 2007 filter
- 2006 (3) Apply 2006 filter
2755 Janelia Publications
Showing 2171-2180 of 2755 resultsThe Drosophila melanogaster sex hierarchy controls sexual differentiation of somatic cells via the activities of the terminal genes in the hierarchy, doublesex (dsx) and fruitless (fru). We have targeted an insertion of GAL4 into the dsx gene, allowing us to visualize dsx-expressing cells in both sexes. Developmentally and as adults, we find that both XX and XY individuals are fine mosaics of cells and tissues that express dsx and/or fruitless (fru(M)), and hence have the potential to sexually differentiate, and those that don’t. Evolutionary considerations suggest such a mosaic expression of sexuality is likely to be a property of other animal species having two sexes. These results have also led to a major revision of our view of how sex-specific functions are regulated by the sex hierarchy in flies. Rather than there being a single regulatory event that governs the activities of all downstream sex determination regulatory genes-turning on Sex lethal (Sxl) RNA splicing activity in females while leaving it turned off in males-there are, in addition, elaborate temporal and spatial transcriptional controls on the expression of the terminal regulatory genes, dsx and fru. Thus tissue-specific aspects of sexual development are jointly specified by post-transcriptional control by Sxl and by the transcriptional controls of dsx and fru expression.
The development of sexually dimorphic morphology and the potential for sexually dimorphic behavior in Drosophila are regulated by the Fruitless (Fru) and Doublesex (Dsx) transcription factors. Several direct targets of Dsx have been identified, but direct Fru targets have not been definitively identified. We show that Drosophila leucine-rich repeat G protein-coupled receptor 3 (Lgr3) is regulated by Fru and Dsx in separate populations of neurons. Lgr3 is a member of the relaxin-receptor family and a receptor for Dilp8, necessary for control of organ growth. Lgr3 expression in the anterior central brain of males is inhibited by the B isoform of Fru, whose DNA binding domain interacts with a short region of an Lgr3 intron. Fru A and C isoform mutants had no observed effect on Lgr3 expression. The female form of Dsx (Dsx(F)) separately up- and down-regulates Lgr3 expression in distinct neurons in the abdominal ganglion through female- and male-specific Lgr3 enhancers. Excitation of neural activity in the Dsx(F)-up-regulated abdominal ganglion neurons inhibits female receptivity, indicating the importance of these neurons for sexual behavior. Coordinated regulation of Lgr3 by Fru and Dsx marks a point of convergence of the two branches of the sex-determination hierarchy.
The brain’s reward systems reinforce behaviors required for species survival, including sex, food consumption, and social interaction. Drugs of abuse co-opt these neural pathways, which can lead to addiction. Here, we used Drosophila melanogaster to investigate the relationship between natural and drug rewards. In males, mating increased, whereas sexual deprivation reduced, neuropeptide F (NPF) levels. Activation or inhibition of the NPF system in turn reduced or enhanced ethanol preference. These results thus link sexual experience, NPF system activity, and ethanol consumption. Artificial activation of NPF neurons was in itself rewarding and precluded the ability of ethanol to act as a reward. We propose that activity of the NPF-NPF receptor axis represents the state of the fly reward system and modifies behavior accordingly.
Sex differences in behaviour exist across the animal kingdom, typically under strong genetic regulation. In Drosophila, previous work has shown that fruitless and doublesex transcription factors identify neurons driving sexually dimorphic behaviour. However, the organisation of dimorphic neurons into functional circuits remains unclear.We now present the connectome of the entire Drosophila male central nervous system. This contains 166,691 neurons spanning the brain and ventral nerve cord, fully proofread and comprehensively annotated including fruitless and doublesex expression and 11,691 cell types. By comparison with a previous female brain connectome, we provide the first comprehensive description of the differences between male and female brains to synaptic resolution. Of 7,319 cross-matched cell types in the central brain, 114 are dimorphic with an additional 262 male- and 69 female-specific (totalling 4.8% of neurons in males and 2.4% in females).This resource enables analysis of full sensory-to-motor circuits underlying complex behaviours as well as the impact of dimorphic elements. Sex-specific and dimorphic neurons are concentrated in higher brain centres while the sensory and motor periphery are largely isomorphic. Within higher centres, male-specific connections are organised into hotspots defined by male-specific neurons or the presence of male-specific arbours on neurons that are otherwise similar between sexes. Numerous circuit switches reroute sensory information to form conserved, antagonistic circuits controlling opposing behaviours.
Sexual dimorphisms are present across brains. Male and female brains contain sets of cell types with differences in cell number, morphology, or synaptic connectivity between the two sexes. These differences are driven by differentially-expressed transcription factors, which set the stage for disparate sexual and social behaviors observed between males and females, such as courtship, aggression, receptivity, and mating. In the Drosophila brain, sexual dimorphisms result from differential expression of two transcription factors, Fruitless (Fru) and Doublesex (Dsx), and genetic reagents driven by enhancers for Fru and Dsx label sexually-dimorphic neurons in both male and female brains. The recent release of the first whole-brain connectome for Drosophila provides a unique opportunity to study the connectivity between these neurons as well as their integration into the larger brain network. Here, we identify 91 putative Fru or Dsx cell types, comprising ~1400 neurons, within the whole-brain connectome, using morphological similarity between electron microscopic (EM) reconstructions and light microscopic (LM) images of known Fru and Dsx neurons. We discover that while Fru and Dsx neurons are highly interconnected, each cell type typically receives more inputs from and sends more outputs to non-Fru/Dsx neurons. We characterize the connectivity in the Fru/Dsx networks to predict the function of cell types not previously characterized, we measure distances to the sensory periphery and uncover multisensory interactions, and we map connections to descending neurons that drive behavior. All Fru and Dsx labels reported here are shared within FlyWire Codex (codex.flywire.ai; gene==Fruitless or Doublesex); this work is a critical first step towards deciphering the neural basis of sexually-dimorphic behaviors and for making comparisons with future connectomes of the male brain.
Animal development is orchestrated by spatio-temporal gene expression programmes that drive precise lineage commitment, proliferation and migration events at the single-cell level, collectively leading to large-scale morphological change and functional specification in the whole organism. Efforts over decades have uncovered two 'seemingly contradictory' mechanisms in gene regulation governing these intricate processes: (i) stochasticity at individual gene regulatory steps in single cells and (ii) highly coordinated gene expression dynamics in the embryo. Here we discuss how these two layers of regulation arise from the molecular and the systems level, and how they might interplay to determine cell fate and to control the complex body plan. We also review recent technological advancements that enable quantitative analysis of gene regulation dynamics at single-cell, single-molecule resolution. These approaches outline next-generation experiments to decipher general principles bridging gaps between molecular dynamics in single cells and robust gene regulations in the embryo.
The mammalian retina conveys the vast majority of information about visual stimuli to two brain regions: the dorsal lateral geniculate nucleus (dLGN) and the superior colliculus (SC). The degree to which retinal ganglion cells (RGCs) send similar or distinct information to the two areas remains unclear despite the important constraints that different patterns of RGC input place on downstream visual processing. To resolve this ambiguity we injected a glycoprotein-deficient rabies virus coding for the expression of a fluorescent protein into the dLGN or SC; rabies virus labeled a smaller fraction of RGCs than lipophilic dyes like DiI but, crucially, did not label RGC axons of passage. ~80% of the RGCs infected by rabies virus injected into the dLGN were co-labeled with DiI injected into the SC, suggesting that many dLGN-projecting RGCs also project to the SC. However, functional characterization of RGCs revealed that the SC receives input from several classes of RGCs that largely avoid the dLGN - in particular, RGCs in which (1) sustained changes in light intensity elicit transient changes in firing rate and/or (2) a small range of stimulus sizes or temporal fluctuations in light intensity elicit robust activity. Taken together, our results illustrate several unexpected asymmetries in the information that the mouse retina conveys to two major downstream targets and suggest that differences in the output of dLGN and SC neurons reflect, at least in part, differences in the functional properties of RGCs that innervate the SC but not the dLGN.
The neocortex contains a multitude of cell types that are segregated into layers and functionally distinct areas. To investigate the diversity of cell types across the mouse neocortex, here we analysed 23,822 cells from two areas at distant poles of the mouse neocortex: the primary visual cortex and the anterior lateral motor cortex. We define 133 transcriptomic cell types by deep, single-cell RNA sequencing. Nearly all types of GABA (γ-aminobutyric acid)-containing neurons are shared across both areas, whereas most types of glutamatergic neurons were found in one of the two areas. By combining single-cell RNA sequencing and retrograde labelling, we match transcriptomic types of glutamatergic neurons to their long-range projection specificity. Our study establishes a combined transcriptomic and projectional taxonomy of cortical cell types from functionally distinct areas of the adult mouse cortex.
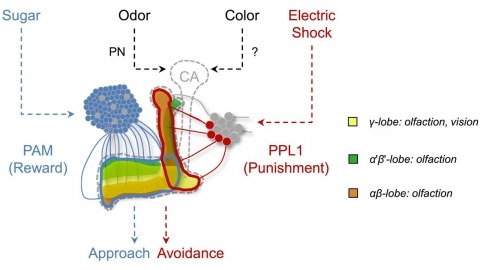
In nature, animals form memories associating reward or punishment with stimuli from different sensory modalities, such as smells and colors. It is unclear, however, how distinct sensory memories are processed in the brain. We established appetitive and aversive visual learning assays for Drosophila that are comparable to the widely used olfactory learning assays. These assays share critical features, such as reinforcing stimuli (sugar reward and electric shock punishment), and allow direct comparison of the cellular requirements for visual and olfactory memories. We found that the same subsets of dopamine neurons drive formation of both sensory memories. Furthermore, distinct yet partially overlapping subsets of mushroom body intrinsic neurons are required for visual and olfactory memories. Thus, our results suggest that distinct sensory memories are processed in a common brain center. Such centralization of related brain functions is an economical design that avoids the repetition of similar circuit motifs.
Light sheet-based fluorescence microscopy (LSFM) is emerging as a powerful imaging technique for the life sciences. LSFM provides an exceptionally high imaging speed, high signal-to-noise ratio, low level of photo-bleaching and good optical penetration depth. This unique combination of capabilities makes light sheet-based microscopes highly suitable for live imaging applications. There is an outstanding potential in applying this technology to the quantitative study of embryonic development. Here, we provide an overview of the different basic implementations of LSFM, review recent technical advances in the field and highlight applications in the context of embryonic development. We conclude with a discussion of promising future directions.