Filter
Associated Lab
- Aso Lab (30) Apply Aso Lab filter
- Betzig Lab (1) Apply Betzig Lab filter
- Bock Lab (2) Apply Bock Lab filter
- Branson Lab (8) Apply Branson Lab filter
- Card Lab (5) Apply Card Lab filter
- Clapham Lab (1) Apply Clapham Lab filter
- Dickson Lab (2) Apply Dickson Lab filter
- Druckmann Lab (1) Apply Druckmann Lab filter
- Fetter Lab (1) Apply Fetter Lab filter
- Funke Lab (1) Apply Funke Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Heberlein Lab (1) Apply Heberlein Lab filter
- Hermundstad Lab (2) Apply Hermundstad Lab filter
- Hess Lab (5) Apply Hess Lab filter
- Jayaraman Lab (6) Apply Jayaraman Lab filter
- Lippincott-Schwartz Lab (1) Apply Lippincott-Schwartz Lab filter
- Looger Lab (2) Apply Looger Lab filter
- O'Shea Lab (1) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Reiser Lab (15) Apply Reiser Lab filter
- Riddiford Lab (1) Apply Riddiford Lab filter
- Romani Lab (1) Apply Romani Lab filter
- Remove Rubin Lab filter Rubin Lab
- Saalfeld Lab (4) Apply Saalfeld Lab filter
- Scheffer Lab (7) Apply Scheffer Lab filter
- Schreiter Lab (1) Apply Schreiter Lab filter
- Simpson Lab (3) Apply Simpson Lab filter
- Singer Lab (1) Apply Singer Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Svoboda Lab (3) Apply Svoboda Lab filter
- Truman Lab (4) Apply Truman Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
- Turner Lab (5) Apply Turner Lab filter
Associated Project Team
- CellMap (1) Apply CellMap filter
- Fly Functional Connectome (4) Apply Fly Functional Connectome filter
- Fly Olympiad (3) Apply Fly Olympiad filter
- FlyEM (11) Apply FlyEM filter
- FlyLight (20) Apply FlyLight filter
- GENIE (1) Apply GENIE filter
- Transcription Imaging (1) Apply Transcription Imaging filter
Associated Support Team
- Project Pipeline Support (1) Apply Project Pipeline Support filter
- Electron Microscopy (4) Apply Electron Microscopy filter
- Invertebrate Shared Resource (9) Apply Invertebrate Shared Resource filter
- Janelia Experimental Technology (1) Apply Janelia Experimental Technology filter
- Management Team (1) Apply Management Team filter
- Primary & iPS Cell Culture (1) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (11) Apply Project Technical Resources filter
- Quantitative Genomics (2) Apply Quantitative Genomics filter
- Scientific Computing Software (12) Apply Scientific Computing Software filter
- Scientific Computing Systems (2) Apply Scientific Computing Systems filter
Publication Date
- 2025 (4) Apply 2025 filter
- 2024 (4) Apply 2024 filter
- 2023 (5) Apply 2023 filter
- 2022 (1) Apply 2022 filter
- 2021 (4) Apply 2021 filter
- 2020 (9) Apply 2020 filter
- 2019 (6) Apply 2019 filter
- 2018 (7) Apply 2018 filter
- 2017 (15) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (16) Apply 2015 filter
- 2014 (8) Apply 2014 filter
- 2013 (5) Apply 2013 filter
- 2012 (7) Apply 2012 filter
- 2011 (3) Apply 2011 filter
- 2010 (2) Apply 2010 filter
- 2009 (1) Apply 2009 filter
- 2008 (2) Apply 2008 filter
- 2007 (2) Apply 2007 filter
- 2006 (1) Apply 2006 filter
105 Janelia Publications
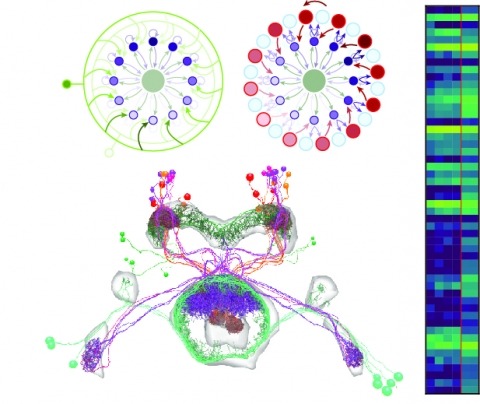
Showing 21-30 of 105 resultsNeural representations of head direction (HD) have been discovered in many species. Theoretical work has proposed that the dynamics associated with these representations are generated, maintained, and updated by recurrent network structures called ring attractors. We evaluated this theorized structure-function relationship by performing electron-microscopy-based circuit reconstruction and RNA profiling of identified cell types in the HD system of Drosophila melanogaster. We identified motifs that have been hypothesized to maintain the HD representation in darkness, update it when the animal turns, and tether it to visual cues. Functional studies provided support for the proposed roles of individual excitatory or inhibitory circuit elements in shaping activity. We also discovered recurrent connections between neuronal arbors with mixed pre- and postsynaptic specializations. Our results confirm that the Drosophila HD network contains the core components of a ring attractor while also revealing unpredicted structural features that might enhance the network's computational power.
Large scientific projects in genomics and astronomy are influential not because they answer any single question but because they enable investigation of continuously arising new questions from the same data-rich sources. Advances in automated mapping of the brain's synaptic connections (connectomics) suggest that the complicated circuits underlying brain function are ripe for analysis. We discuss benefits of mapping a mouse brain at the level of synapses.
The neural circuits responsible for animal behavior remain largely unknown. We summarize new methods and present the circuitry of a large fraction of the brain of the fruit fly . Improved methods include new procedures to prepare, image, align, segment, find synapses in, and proofread such large data sets. We define cell types, refine computational compartments, and provide an exhaustive atlas of cell examples and types, many of them novel. We provide detailed circuits consisting of neurons and their chemical synapses for most of the central brain. We make the data public and simplify access, reducing the effort needed to answer circuit questions, and provide procedures linking the neurons defined by our analysis with genetic reagents. Biologically, we examine distributions of connection strengths, neural motifs on different scales, electrical consequences of compartmentalization, and evidence that maximizing packing density is an important criterion in the evolution of the fly's brain.
Nervous systems contain sensory neurons, local neurons, projection neurons, and motor neurons. To understand how these building blocks form whole circuits, we must distil these broad classes into neuronal cell types and describe their network connectivity. Using an electron micrograph dataset for an entire Drosophila melanogaster brain, we reconstruct the first complete inventory of olfactory projections connecting the antennal lobe, the insect analog of the mammalian olfactory bulb, to higher-order brain regions in an adult animal brain. We then connect this inventory to extant data in the literature, providing synaptic-resolution "holotypes" both for heavily investigated and previously unknown cell types. Projection neurons are approximately twice as numerous as reported by light level studies; cell types are stereotyped, but not identical, in cell and synapse numbers between brain hemispheres. The lateral horn, the insect analog of the mammalian cortical amygdala, is the main target for this olfactory information and has been shown to guide innate behavior. Here, we find new connectivity motifs, including axo-axonic connectivity between projection neurons, feedback, and lateral inhibition of these axons by a large population of neurons, and the convergence of different inputs, including non-olfactory inputs and memory-related feedback onto third-order olfactory neurons. These features are less prominent in the mushroom body calyx, the insect analog of the mammalian piriform cortex and a center for associative memory. Our work provides a complete neuroanatomical platform for future studies of the adult Drosophila olfactory system.
Different types of Drosophila dopaminergic neurons (DANs) reinforce memories of unique valence and provide state-dependent motivational control [1]. Prior studies suggest that the compartment architecture of the mushroom body (MB) is the relevant resolution for distinct DAN functions [2, 3]. Here we used a recent electron microscope volume of the fly brain [4] to reconstruct the fine anatomy of individual DANs within three MB compartments. We find the 20 DANs of the γ5 compartment, at least some of which provide reward teaching signals, can be clustered into 5 anatomical subtypes that innervate different regions within γ5. Reconstructing 821 upstream neurons reveals input selectivity, supporting the functional relevance of DAN sub-classification. Only one PAM-γ5 DAN subtype (γ5fb) receives direct recurrent input from γ5β’2a mushroom body output neurons (MBONs) and behavioral experiments distinguish a role for these DANs in memory revaluation from those reinforcing sugar memory. Other DAN subtypes receive major, and potentially reinforcing, inputs from putative gustatory interneurons or lateral horn neurons, which can also relay indirect feedback from the MB. We similarly reconstructed the single aversively reinforcing PPL1-γ1pedc DAN. The γ1pedc DAN inputs are mostly different to those of γ5 DANs and are clustered onto distinct branches of its dendritic tree, presumably separating its established roles in aversive reinforcement and appetitive motivation [5, 6]. Additional tracing identified neurons that provide broad input to γ5, β’2a and γ1pedc DANs suggesting that distributed DAN populations can be coordinately regulated. These connectomic and behavioral analyses therefore reveal additional complexity of dopaminergic reinforcement circuits between and within MB compartments.
The Mushroom Body (MB) is the primary location of stored associative memories in the Drosophila brain. We discuss recent advances in understanding the MB's neuronal circuits made using advanced light microscopic methods and cell-type-specific genetic tools. We also review how the compartmentalized nature of the MB's organization allows this brain area to form and store memories with widely different dynamics.
The anatomy of many neural circuits is being characterized with increasing resolution, but their molecular properties remain mostly unknown. Here, we characterize gene expression patterns in distinct neural cell types of the visual system using genetic lines to access individual cell types, the TAPIN-seq method to measure their transcriptomes, and a probabilistic method to interpret these measurements. We used these tools to build a resource of high-resolution transcriptomes for 100 driver lines covering 67 cell types, available at http://www.opticlobe.com. Combining these transcriptomes with recently reported connectomes helps characterize how information is transmitted and processed across a range of scales, from individual synapses to circuit pathways. We describe examples that include identifying neurotransmitters, including cases of apparent co-release, generating functional hypotheses based on receptor expression, as well as identifying strong commonalities between different cell types.
Animals employ diverse learning rules and synaptic plasticity dynamics to record temporal and statistical information about the world. However, the molecular mechanisms underlying this diversity are poorly understood. The anatomically defined compartments of the insect mushroom body function as parallel units of associative learning, with different learning rates, memory decay dynamics and flexibility (Aso & Rubin 2016). Here we show that nitric oxide (NO) acts as a neurotransmitter in a subset of dopaminergic neurons in . NO's effects develop more slowly than those of dopamine and depend on soluble guanylate cyclase in postsynaptic Kenyon cells. NO acts antagonistically to dopamine; it shortens memory retention and facilitates the rapid updating of memories. The interplay of NO and dopamine enables memories stored in local domains along Kenyon cell axons to be specialized for predicting the value of odors based only on recent events. Our results provide key mechanistic insights into how diverse memory dynamics are established in parallel memory systems.
Animals exhibit innate behaviours to a variety of sensory stimuli including olfactory cues. In , one higher olfactory centre, the lateral horn (LH), is implicated in innate behaviour. However, our structural and functional understanding of the LH is scant, in large part due to a lack of sparse neurogenetic tools for this region. We generate a collection of split-GAL4 driver lines providing genetic access to 82 LH cell types. We use these to create an anatomical and neurotransmitter map of the LH and link this to EM connectomics data. We find ~30% of LH projections converge with outputs from the mushroom body, site of olfactory learning and memory. Using optogenetic activation, we identify LH cell types that drive changes in valence behavior or specific locomotor programs. In summary, we have generated a resource for manipulating and mapping LH neurons, providing new insights into the circuit basis of innate and learned olfactory behavior.
Starting a new research campus is a leap of faith. Only later, in the full measure of time, is it possible to take stock of what has worked and what could have been done better or differently. The Janelia Research Campus opened its doors 12 years ago. What has it achieved? What has it taught us? And where does Janelia go from here?