Filter
Associated Lab
- Ahrens Lab (7) Apply Ahrens Lab filter
- Druckmann Lab (2) Apply Druckmann Lab filter
- Dudman Lab (1) Apply Dudman Lab filter
- Freeman Lab (2) Apply Freeman Lab filter
- Harris Lab (7) Apply Harris Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Hess Lab (2) Apply Hess Lab filter
- Jayaraman Lab (10) Apply Jayaraman Lab filter
- Karpova Lab (2) Apply Karpova Lab filter
- Keller Lab (5) Apply Keller Lab filter
- Lavis Lab (8) Apply Lavis Lab filter
- Leonardo Lab (4) Apply Leonardo Lab filter
- Remove Looger Lab filter Looger Lab
- Podgorski Lab (6) Apply Podgorski Lab filter
- Rubin Lab (2) Apply Rubin Lab filter
- Schreiter Lab (24) Apply Schreiter Lab filter
- Simpson Lab (1) Apply Simpson Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Sternson Lab (2) Apply Sternson Lab filter
- Svoboda Lab (20) Apply Svoboda Lab filter
- Tervo Lab (1) Apply Tervo Lab filter
- Tillberg Lab (1) Apply Tillberg Lab filter
- Turner Lab (1) Apply Turner Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2024 (2) Apply 2024 filter
- 2023 (5) Apply 2023 filter
- 2022 (7) Apply 2022 filter
- 2021 (11) Apply 2021 filter
- 2020 (7) Apply 2020 filter
- 2019 (15) Apply 2019 filter
- 2018 (8) Apply 2018 filter
- 2017 (6) Apply 2017 filter
- 2016 (10) Apply 2016 filter
- 2015 (9) Apply 2015 filter
- 2014 (11) Apply 2014 filter
- 2013 (10) Apply 2013 filter
- 2012 (13) Apply 2012 filter
- 2011 (7) Apply 2011 filter
- 2010 (7) Apply 2010 filter
- 2009 (7) Apply 2009 filter
- 2008 (3) Apply 2008 filter
Type of Publication
138 Publications
Showing 81-90 of 138 resultsStaphylococcus aureus responds to changing extracellular environments in part by adjusting its proteome through alterations of transcriptional priorities and selective degradation of the preexisting pool of proteins. In Bacillus subtilis, the proteolytic adaptor protein MecA has been shown to play a role in assisting with the proteolytic degradation of proteins involved in competence and the oxidative stress response. However, the targets of TrfA, the MecA homolog in S. aureus, have not been well characterized. In this work, we investigated how TrfA assists chaperones and proteases to regulate the proteolysis of several classes of proteins in S. aureus. By fusing the last 3 amino acids of the SsrA degradation tag to Venus, a rapidly folding yellow fluorescent protein, we obtained both fluorescence-based and Western blot assay-based evidence that TrfA and ClpCP are the adaptor and protease, respectively, responsible for the degradation of the SsrA-tagged protein in S. aureus. Notably, the impact of TrfA on degradation was most prominent during late log phase and early stationary phase, due in part to a combination of transcriptional regulation and proteolytic degradation of TrfA by ClpCP. We also characterized the temporal transcriptional regulation governing TrfA activity, wherein Spx, a redox-sensitive transcriptional regulator degraded by ClpXP, activates trfA transcription while repressing its own promoter. Finally, the scope of TrfA-mediated proteolysis was expanded by identifying TrfA as the adaptor that works with ClpCP to degrade antitoxins in S. aureus. Together, these results indicate that the adaptor TrfA adds temporal nuance to protein degradation by ClpCP in S. aureus.
Genetically-encoded calcium indicators (GECIs) facilitate imaging activity of genetically defined neuronal populations in vivo. The high intracellular GECI concentrations required for in vivo imaging are usually achieved by viral gene transfer using adeno-associated viruses. Transgenic expression of GECIs promises important advantages, including homogeneous, repeatable, and stable expression without the need for invasive virus injections. Here we present the generation and characterization of transgenic mice expressing the GECIs GCaMP6s or GCaMP6f under the Thy1 promoter. We quantified GCaMP6 expression across brain regions and neurons and compared to other transgenic mice and AAV-mediated expression. We tested three mouse lines for imaging in the visual cortex in vivo and compared their performance to mice injected with AAV expressing GCaMP6. Furthermore, we show that GCaMP6 Thy1 transgenic mice are useful for long-term, high-sensitivity imaging in behaving mice.
Integrin alpha M (ITGAM; CD11b) is a component of the macrophage-1 antigen complex, which mediates leukocyte adhesion, migration and phagocytosis as part of the immune system. We previously identified a missense polymorphism, rs1143679 (R77H), strongly associated with systemic lupus erythematosus (SLE). However, the molecular mechanisms of this variant are incompletely understood. A meta-analysis of published and novel data on 28 439 individuals with European, African, Hispanic and Asian ancestries reinforces genetic association between rs1143679 and SLE [Pmeta = 3.60 × 10(-90), odds ratio (OR) = 1.76]. Since rs1143679 is in the most active region of chromatin regulation and transcription factor binding in ITGAM, we quantitated ITGAM RNA and surface protein levels in monocytes from patients with each rs1143679 genotype. We observed that transcript levels significantly decreased for the risk allele ('A') relative to the non-risk allele ('G'), in a dose-dependent fashion: ('AA' < 'AG' < 'GG'). CD11b protein levels in patients' monocytes were directly correlated with RNA levels. Strikingly, heterozygous individuals express much lower (average 10- to 15-fold reduction) amounts of the 'A' transcript than 'G' transcript. We found that the non-risk sequence surrounding rs1143679 exhibits transcriptional enhancer activity in vivo and binds to Ku70/80, NFKB1 and EBF1 in vitro, functions that are significantly reduced with the risk allele. Mutant CD11b protein shows significantly reduced binding to fibrinogen and vitronectin, relative to non-risk, both in purified protein and in cellular models. This two-pronged contribution (nucleic acid- and protein-level) of the rs1143679 risk allele to decreasing ITGAM activity provides insight into the molecular mechanisms of its potent association with SLE.
The mammalian vomeronasal organ encodes pheromone information about gender, reproductive status, genetic background and individual differences. It remains unknown how pheromone information interacts to trigger innate behaviors. In this study, we identify vomeronasal receptors responsible for detecting female pheromones. A sub-group of V1re clade members recognizes gender-identifying cues in female urine. Multiple members of the V1rj clade are cognate receptors for urinary estrus signals, as well as for sulfated estrogen (SE) compounds. In both cases, the same cue activates multiple homologous receptors, suggesting redundancy in encoding female pheromone cues. Neither gender-specific cues nor SEs alone are sufficient to promote courtship behavior in male mice, whereas robust courtship behavior can be induced when the two cues are applied together. Thus, integrated action of different female cues is required in pheromone-triggered mating behavior. These results suggest a gating mechanism in the vomeronasal circuit in promoting specific innate behavior.DOI: http://dx.doi.org/10.7554/eLife.03025.001.
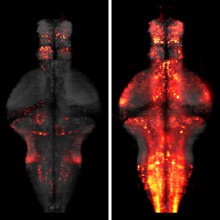
The processing of sensory input and the generation of behavior involves large networks of neurons, which necessitates new technology for recording from many neurons in behaving animals. In the larval zebrafish, light-sheet microscopy can be used to record the activity of almost all neurons in the brain simultaneously at single-cell resolution. Existing implementations, however, cannot be combined with visually driven behavior because the light sheet scans over the eye, interfering with presentation of controlled visual stimuli. Here we describe a system that overcomes the confounding eye stimulation through the use of two light sheets and combines whole-brain light-sheet imaging with virtual reality for fictively behaving larval zebrafish.
Understanding brain function requires monitoring and interpreting the activity of large networks of neurons during behavior. Advances in recording technology are greatly increasing the size and complexity of neural data. Analyzing such data will pose a fundamental bottleneck for neuroscience. We present a library of analytical tools called Thunder built on the open-source Apache Spark platform for large-scale distributed computing. The library implements a variety of univariate and multivariate analyses with a modular, extendable structure well-suited to interactive exploration and analysis development. We demonstrate how these analyses find structure in large-scale neural data, including whole-brain light-sheet imaging data from fictively behaving larval zebrafish, and two-photon imaging data from behaving mouse. The analyses relate neuronal responses to sensory input and behavior, run in minutes or less and can be used on a private cluster or in the cloud. Our open-source framework thus holds promise for turning brain activity mapping efforts into biological insights.
Retinal bipolar cells (BCs) transmit visual signals in parallel channels from the outer to the inner retina, where they provide glutamatergic inputs to specific networks of amacrine and ganglion cells. Intricate network computation at BC axon terminals has been proposed as a mechanism for complex network computation, such as direction selectivity, but direct knowledge of the receptive field property and the synaptic connectivity of the axon terminals of various BC types is required in order to understand the role of axonal computation by BCs. The present study tested the essential assumptions of the presynaptic model of direction selectivity at axon terminals of three functionally distinct BC types that ramify in the direction-selective strata of the mouse retina. Results from two-photon Ca2+ imaging, optogenetic stimulation, and dual patch-clamp recording demonstrated that (1) CB5 cells do not receive fast GABAergic synaptic feedback from starburst amacrine cells (SACs), (2) light-evoked and spontaneous Ca2+ responses are well coordinated among various local regions of CB5 axon terminals, (3) CB5 axon terminals are not directionally selective, (4) CB5 cells consist of two novel functional subtypes with distinct receptive field structures, (5) CB7 cells provide direct excitatory synaptic inputs to, but receive no direct GABAergic synaptic feedback from SACs, and (6) CB7 axon terminals are not directionally selective either. These findings help to simplify models of direction selectivity by ruling out complex computation at BC terminals. They also show that CB5 comprises two functional subclasses of BCs.
A fundamental question in sensory neuroscience is how parallel processing is implemented at the level of molecular and circuit mechanisms. In the retina, it has been proposed that distinct OFF cone bipolar cell types generate fast/transient and slow/sustained pathways by the differential expression of AMPA- and kainate-type glutamate receptors, respectively. However, the functional significance of these receptors in the intact circuit during light stimulation remains unclear. Here, we measured glutamate release from mouse bipolar cells by two-photon imaging of a glutamate sensor (iGluSnFR) expressed on postsynaptic amacrine and ganglion cell dendrites. In both transient and sustained OFF layers, cone-driven glutamate release from bipolar cells was blocked by antagonists to kainate receptors but not AMPA receptors. Electrophysiological recordings from bipolar and ganglion cells confirmed the essential role of kainate receptors for signaling in both transient and sustained OFF pathways. Kainate receptors mediated responses to contrast modulation up to 20 Hz. Light-evoked responses in all mouse OFF bipolar pathways depend on kainate, not AMPA, receptors.
The spatiotemporal activities of astrocyte Ca(2+) signaling in mature neuronal circuits remain unclear. We used genetically encoded Ca(2+) and glutamate indicators as well as pharmacogenetic and electrical control of neurotransmitter release to explore astrocyte activity in the hippocampal mossy fiber pathway. Our data revealed numerous localized, spontaneous Ca(2+) signals in astrocyte branches and territories, but these were not driven by neuronal activity or glutamate. Moreover, evoked astrocyte Ca(2+) signaling changed linearly with the number of mossy fiber action potentials. Under these settings, astrocyte responses were global, suppressed by neurotransmitter clearance, and mediated by glutamate and GABA. Thus, astrocyte engagement in the fully developed mossy fiber pathway was slow and territorial, contrary to that frequently proposed for astrocytes within microcircuits. We show that astrocyte Ca(2+) signaling functionally segregates large volumes of neuropil and that these transients are not suited for responding to, or regulating, single synapses in the mossy fiber pathway.
Recent reports have associated NCF2, encoding a core component of the multi-protein NADPH oxidase (NADPHO), with systemic lupus erythematosus (SLE) susceptibility in individuals of European ancestry. To identify ethnicity-specific and -robust variants within NCF2, we assessed 145 SNPs in and around the NCF2 gene in 5325 cases and 21 866 controls of European-American (EA), African-American (AA), Hispanic (HS) and Korean (KR) ancestry. Subsequent imputation, conditional, haplotype and bioinformatic analyses identified seven potentially functional SLE-predisposing variants. Association with non-synonymous rs17849502, previously reported in EA, was detected in EA, HS and AA (P(EA) = 1.01 × 10(-54), PHS = 3.68 × 10(-10), P(AA) = 0.03); synonymous rs17849501 was similarly significant. These SNPs were monomorphic in KR. Novel associations were detected with coding variants at rs35937854 in AA (PAA = 1.49 × 10(-9)), and rs13306575 in HS and KR (P(HS) = 7.04 × 10(-7), P(KR) = 3.30 × 10(-3)). In KR, a 3-SNP haplotype was significantly associated (P = 4.20 × 10(-7)), implying that SLE predisposing variants were tagged. Significant SNP-SNP interaction (P = 0.02) was detected between rs13306575 and rs17849502 in HS, and a dramatically increased risk (OR = 6.55) with a risk allele at each locus. Molecular modeling predicts that these non-synonymous mutations could disrupt NADPHO complex assembly. The risk allele of rs17849501, located in a conserved transcriptional regulatory region, increased reporter gene activity, suggesting in vivo enhancer function. Our results not only establish allelic heterogeneity within NCF2 associated with SLE, but also emphasize the utility of multi-ethnic cohorts to identify predisposing variants explaining additional phenotypic variance ('missing heritability') of complex diseases like SLE.