Filter
Associated Lab
- Ahrens Lab (4) Apply Ahrens Lab filter
- Betzig Lab (1) Apply Betzig Lab filter
- Beyene Lab (1) Apply Beyene Lab filter
- Druckmann Lab (1) Apply Druckmann Lab filter
- Harris Lab (4) Apply Harris Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Jayaraman Lab (9) Apply Jayaraman Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Keller Lab (1) Apply Keller Lab filter
- Lavis Lab (8) Apply Lavis Lab filter
- Leonardo Lab (1) Apply Leonardo Lab filter
- Liu (Zhe) Lab (1) Apply Liu (Zhe) Lab filter
- Looger Lab (24) Apply Looger Lab filter
- Podgorski Lab (5) Apply Podgorski Lab filter
- Rubin Lab (1) Apply Rubin Lab filter
- Schreiter Lab (67) Apply Schreiter Lab filter
- Stringer Lab (1) Apply Stringer Lab filter
- Svoboda Lab (13) Apply Svoboda Lab filter
- Tillberg Lab (1) Apply Tillberg Lab filter
- Turner Lab (4) Apply Turner Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2025 (3) Apply 2025 filter
- 2024 (5) Apply 2024 filter
- 2023 (6) Apply 2023 filter
- 2021 (1) Apply 2021 filter
- 2020 (5) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (4) Apply 2018 filter
- 2017 (4) Apply 2017 filter
- 2016 (2) Apply 2016 filter
- 2015 (4) Apply 2015 filter
- 2013 (7) Apply 2013 filter
- 2012 (2) Apply 2012 filter
- 2011 (3) Apply 2011 filter
- 2010 (2) Apply 2010 filter
- 2009 (3) Apply 2009 filter
- 2008 (3) Apply 2008 filter
- 2007 (3) Apply 2007 filter
- 2006 (2) Apply 2006 filter
- 2003 (1) Apply 2003 filter
- 2001 (2) Apply 2001 filter
- 1999 (1) Apply 1999 filter
Type of Publication
67 Publications
Showing 21-30 of 67 resultsImaging changes in membrane potential using genetically encoded fluorescent voltage indicators (GEVIs) has great potential for monitoring neuronal activity with high spatial and temporal resolution. Brightness and photostability of fluorescent proteins and rhodopsins have limited the utility of existing GEVIs. We engineered a novel GEVI, "Voltron", that utilizes bright and photostable synthetic dyes instead of protein-based fluorophores, extending the combined duration of imaging and number of neurons imaged simultaneously by more than tenfold relative to existing GEVIs. We used Voltron for in vivo voltage imaging in mice, zebrafish, and fruit flies. In mouse cortex, Voltron allowed single-trial recording of spikes and subthreshold voltage signals from dozens of neurons simultaneously, over 15 min of continuous imaging. In larval zebrafish, Voltron enabled the precise correlation of spike timing with behavior.
Point-scanning two-photon microscopy enables high-resolution imaging within scattering specimens such as the mammalian brain, but sequential acquisition of voxels fundamentally limits imaging speed. We developed a two-photon imaging technique that scans lines of excitation across a focal plane at multiple angles and uses prior information to recover high-resolution images at over 1.4 billion voxels per second. Using a structural image as a prior for recording neural activity, we imaged visually-evoked and spontaneous glutamate release across hundreds of dendritic spines in mice at depths over 250 microns and frame-rates over 1 kHz. Dendritic glutamate transients in anaesthetized mice are synchronized within spatially-contiguous domains spanning tens of microns at frequencies ranging from 1-100 Hz. We demonstrate high-speed recording of acetylcholine and calcium sensors, 3D single-particle tracking, and imaging in densely-labeled cortex. Our method surpasses limits on the speed of raster-scanned imaging imposed by fluorescence lifetime.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The resulting jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and may allow tracking larger populations of neurons using two-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
We report an intensiometric, near-infrared fluorescent, genetically encoded calcium ion (Ca) indicator (GECI) with excitation and emission maxima at 678 and 704 nm, respectively. This GECI, designated NIR-GECO1, enables imaging of Ca transients in cultured mammalian cells and brain tissue with sensitivity comparable to that of currently available visible-wavelength GECIs. We demonstrate that NIR-GECO1 opens up new vistas for multicolor Ca imaging in combination with other optogenetic indicators and actuators.
Marking functionally distinct neuronal ensembles with high spatiotemporal resolution is a key challenge in systems neuroscience. We recently introduced CaMPARI, an engineered fluorescent protein whose green-to-red photoconversion depends on simultaneous light exposure and elevated calcium, which enabled marking active neuronal populations with single-cell and subsecond resolution. However, CaMPARI (CaMPARI1) has several drawbacks, including background photoconversion in low calcium, slow kinetics and reduced fluorescence after chemical fixation. In this work, we develop CaMPARI2, an improved sensor with brighter green and red fluorescence, faster calcium unbinding kinetics and decreased photoconversion in low calcium conditions. We demonstrate the improved performance of CaMPARI2 in mammalian neurons and in vivo in larval zebrafish brain and mouse visual cortex. Additionally, we herein develop an immunohistochemical detection method for specific labeling of the photoconverted red form of CaMPARI. The anti-CaMPARI-red antibody provides strong labeling that is selective for photoconverted CaMPARI in activated neurons in rodent brain tissue.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics, and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and tracking large populations of neurons using 2-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
We have developed a series of yellow genetically encoded Ca indicators for optical imaging (Y-GECOs) with inverted responses to Ca and apparent dissociation constants (K') ranging from 25 to 2400 nM. To demonstrate the utility of this affinity series of Ca indicators, we expressed the four highest affinity variants (K's = 25, 63, 121, and 190 nM) in the Drosophila medulla intrinsic neuron Mi1. Hyperpolarization of Mi1 by optogenetic stimulation of the laminar monopolar neuron L1 produced a decrease in intracellular Ca in layers 8-10, and a corresponding increase in Y-GECO fluorescence. These experiments revealed that lower K' was associated with greater increases in fluorescence, but longer delays to reach the maximum signal change due to slower off-rate kinetics.
BACKGROUND: Genetically encoded calcium ion (Ca2+) indicators (GECIs) are indispensable tools for measuring Ca2+ dynamics and neuronal activities in vitro and in vivo. Red fluorescent protein (RFP)-based GECIs have inherent advantages relative to green fluorescent protein-based GECIs due to the longer wavelength light used for excitation. Longer wavelength light is associated with decreased phototoxicity and deeper penetration through tissue. Red GECI can also enable multicolor visualization with blue- or cyan-excitable fluorophores. RESULTS: Here we report the development, structure, and validation of a new RFP-based GECI, K-GECO1, based on a circularly permutated RFP derived from the sea anemone Entacmaea quadricolor. We have characterized the performance of K-GECO1 in cultured HeLa cells, dissociated neurons, stem-cell-derived cardiomyocytes, organotypic brain slices, zebrafish spinal cord in vivo, and mouse brain in vivo. CONCLUSION: K-GECO1 is the archetype of a new lineage of GECIs based on the RFP eqFP578 scaffold. It offers high sensitivity and fast kinetics, similar or better than those of current state-of-the-art indicators, with diminished lysosomal accumulation and minimal blue-light photoactivation. Further refinements of the K-GECO1 lineage could lead to further improved variants with overall performance that exceeds that of the most highly optimized red GECIs.
The present disclosure provides, inter alia, genetically encoded recombinant peptide biosensors comprising analyte-binding framework portions and signaling portions, wherein the signaling portions are present within the framework portions at sites or amino acid positions that undergo a conformational change upon interaction of the framework portion with an analyte.
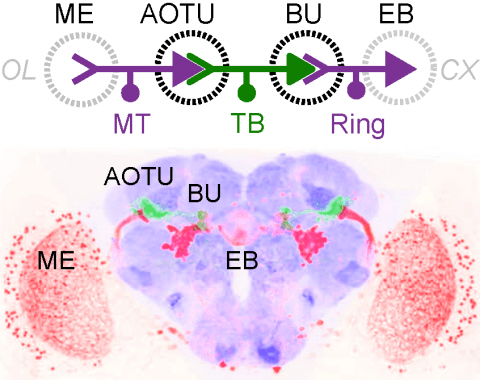
Many animals orient using visual cues, but how a single cue is selected from among many is poorly understood. Here we show that Drosophila ring neurons—central brain neurons implicated in navigation—display visual stimulus selection. Using in vivo two-color two-photon imaging with genetically encoded calcium indicators, we demonstrate that individual ring neurons inherit simple-cell-like receptive fields from their upstream partners. Stimuli in the contralateral visual field suppressed responses to ipsilateral stimuli in both populations. Suppression strength depended on when and where the contralateral stimulus was presented, an effect stronger in ring neurons than in their upstream inputs. This history-dependent effect on the temporal structure of visual responses, which was well modeled by a simple biphasic filter, may determine how visual references are selected for the fly's internal compass. Our approach highlights how two-color calcium imaging can help identify and localize the origins of sensory transformations across synaptically connected neural populations.