Andy Moore thought his paper was ready to submit. As a graduate student, he’d used a high-tech microscope housed in Janelia’s Advanced Imaging Center to capture new details on mitochondria, bean-shaped structures that make energy for cells.
But when Moore arrived at Janelia months later for a full-time position as a postdoc, he took another look under a different powerful microscope—and changed his mind. “I could actually see this entire world that was way more complex than how we’d described it,” Moore says. “Once I saw that, it was like a compulsion--I couldn't not pursue it.”
Most research on cell division has focused on what happens to the DNA in the cell’s nucleus. Cells must duplicate their DNA, then divide the genetic material between the two daughter cells. But mitochondria also contain their own DNA. And since cells can’t generate new mitochondria from scratch, they must somehow also split up their mitochondria when they divide.
More than a century ago, scientists peering through basic light microscopes had noted mitochondria floating around during cell division. “We kind of assumed it was a passive process,” Moore says—that mitochondria, just by chance, end up evenly distributed between new cells.
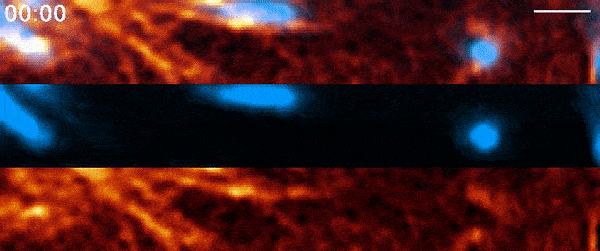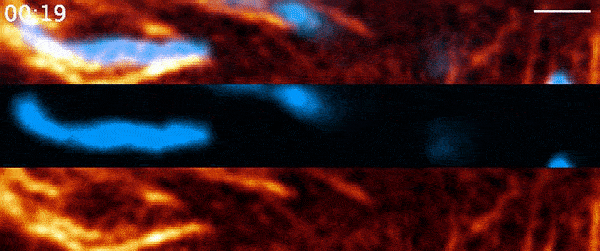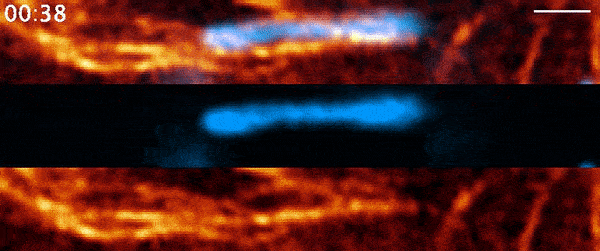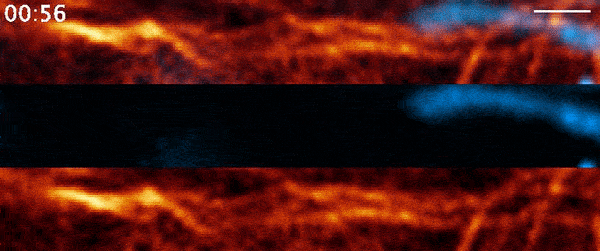
Instead, when Moore used 21st-century tools, he found the process was far more active than anyone had suspected. It involves a protein called actin—a major component of the cell’s skeleton that forms long filaments to support cell shape and move organelles around. Comet-shaped actin structures propel mitochondria around the cell during division, shuffling the mitochondria and then dealing them out like a deck of cards. Moore and his colleagues describe the finding in a paper published March 3, 2021, in the journal Nature.
“People have seen actin comet tails pushing around intracellular pathogens like bacteria, but nobody has described it before with mitochondria,” Moore says.
For Moore, the work began several years before he started at Janelia. In 2018, he was a PhD student at the University of Pennsylvania, working in the lab of Erika Holzbaur. The lab was investigating this question of how cells distribute their mitochondria when they divide. To help solve the mystery, they submitted a proposal to Janelia’s Advanced Imaging Center.
Researchers from outside institutions can apply to visit Janelia and use the cutting-edge microscopes housed at the AIC—tools that are too specialized or too expensive to be commonplace in individual labs.
“Their proposal presented a biological phenomenon that was pretty provocative,” says Leong Chew, director of the AIC. “And we thought, ‘wow, it would be very cool to see how this plays out.’”
The lattice light sheet microscope was exactly what Moore’s team needed to track mitochondria through the process of cell division. It can image living cells, at super-high resolution. “We not only could see what was going on in the cells, but we knew we could image them without destroying the sample or altering the biology,” Moore says.
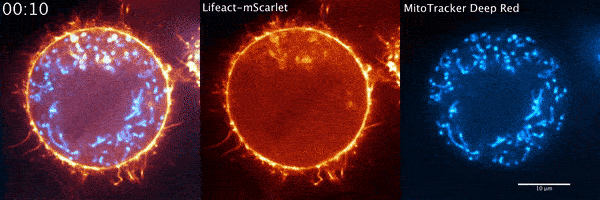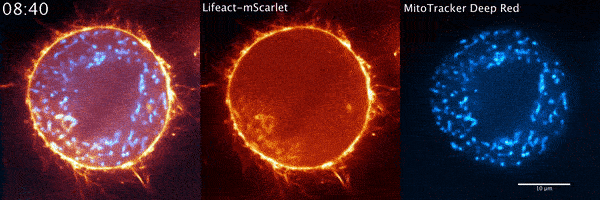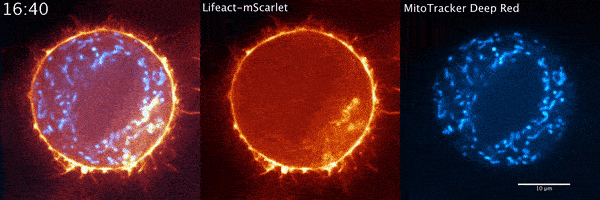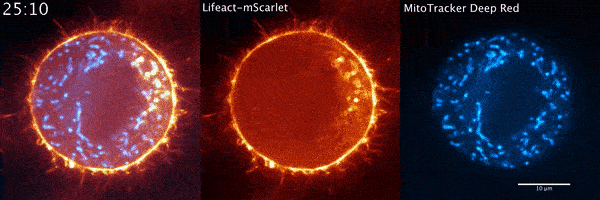
During their several-week stint at Janelia, Moore and his Penn colleagues got what they were hoping to find quickly. The microscope data showed a wave of actin sweeping around the cell and positioning the mitochondria so that when the cell divided, each new cell would get half of the mitochondria. The story seemed complete.
But when Moore arrived full time at Janelia later that year for his postdoc and started going through the data again, it soon became clear that there was more going on. He used a higher-resolution microscope, the Airyscan confocal microscope, to take a second look. The actin wasn’t just moving in a wave, he noted. It was forming comet-like trails that were pushing mitochondria around the cell in bursts—mixing up the mitochondria, not just positioning them.
Mitochondria would start out surrounded by cloud-like shells of actin, Moore says. Then, the symmetry would break. A gap would form in each cloud, and the mitochondria would shoot through those gaps like bubbles bursting, now followed by streaking tails of actin.
“The weirdest part about it was that they were randomly oriented,” Moore says. “So even though this wave was moving in one direction, when you got this transition from clouds to comets, the mitochondria would shoot in all directions.”
Such active mixing could be important for avoiding the effects of localized damage to a cell, Moore suggests. Mitochondria in one part of the cell might have experienced different conditions than mitochondria elsewhere in the cell. With this shuffling process, any damaged mitochondria will be evenly distributed between daughter cells, rather than disproportionally handed off to one cell.
###
Citation
Andrew S. Moore, Stephen M. Coscia, Cory L. Simpson, Fabian E. Ortega, Eric C. Wait, John M. Heddleston, Jeffrey J. Nirschl, Christopher J. Obara, Pedro Guedes-Dias, C. Alexander Boecker, Teng-Leong Chew, Julie A. Theriot, Jennifer Lippincott-Schwartz, & Erika L. F. Holzbaur. “Actin cables and comet tails organize mitochondrial networks in mitosis,” Nature, Published online March 3, 2021. doi:10.1038/s41586-021-03309-5.




