Filter
Associated Lab
- Darshan Lab (1) Apply Darshan Lab filter
- Dickson Lab (3) Apply Dickson Lab filter
- Fitzgerald Lab (1) Apply Fitzgerald Lab filter
- Hermundstad Lab (2) Apply Hermundstad Lab filter
- Jayaraman Lab (2) Apply Jayaraman Lab filter
- Romani Lab (1) Apply Romani Lab filter
- Turner Lab (1) Apply Turner Lab filter
Associated Project Team
Associated Support Team
- Remove Invertebrate Shared Resource filter Invertebrate Shared Resource
- Remove Janelia Experimental Technology filter Janelia Experimental Technology
- Molecular Genomics (2) Apply Molecular Genomics filter
- Project Technical Resources (4) Apply Project Technical Resources filter
6 Janelia Publications
Showing 1-6 of 6 resultsForaging animals must use decision-making strategies that dynamically adapt to the changing availability of rewards in the environment. A wide diversity of animals do this by distributing their choices in proportion to the rewards received from each option, Herrnstein’s operant matching law. Theoretical work suggests an elegant mechanistic explanation for this ubiquitous behavior, as operant matching follows automatically from simple synaptic plasticity rules acting within behaviorally relevant neural circuits. However, no past work has mapped operant matching onto plasticity mechanisms in the brain, leaving the biological relevance of the theory unclear. Here we discovered operant matching in Drosophila and showed that it requires synaptic plasticity that acts in the mushroom body and incorporates the expectation of reward. We began by developing a novel behavioral paradigm to measure choices from individual flies as they learn to associate odor cues with probabilistic rewards. We then built a model of the fly mushroom body to explain each fly’s sequential choice behavior using a family of biologically-realistic synaptic plasticity rules. As predicted by past theoretical work, we found that synaptic plasticity rules could explain fly matching behavior by incorporating stimulus expectations, reward expectations, or both. However, by optogenetically bypassing the representation of reward expectation, we abolished matching behavior and showed that the plasticity rule must specifically incorporate reward expectations. Altogether, these results reveal the first synaptic level mechanisms of operant matching and provide compelling evidence for the role of reward expectation signals in the fly brain.
Internal representations are thought to support the generation of flexible, long-timescale behavioral patterns in both animals and artificial agents. Here, we present a novel conceptual framework for how Drosophila use their internal representation of head direction to maintain preferred headings in their surroundings, and how they learn to modify these preferences in the presence of selective thermal reinforcement. To develop the framework, we analyzed flies’ behavior in a classical operant visual learning paradigm and found that they use stochastically generated fixations and directed turns to express their heading preferences. Symmetries in the visual scene used in the paradigm allowed us to expose how flies’ probabilistic behavior in this setting is tethered to their head direction representation. We describe how flies’ ability to quickly adapt their behavior to the rules of their environment may rest on a behavioral policy whose parameters are flexible but whose form is genetically encoded in the structure of their circuits. Many of the mechanisms we outline may also be relevant for rapidly adaptive behavior driven by internal representations in other animals, including mammals.
Choosing a mate is one of the most consequential decisions a female will make during her lifetime. A female fly signals her willingness to mate by opening her vaginal plates, allowing a courting male to copulate. Vaginal plate opening (VPO) occurs in response to the male courtship song and is dependent on the mating status of the female. How these exteroceptive (song) and interoceptive (mating status) inputs are integrated to regulate VPO remains unknown. Here we characterize the neural circuitry that implements mating decisions in the brain of female Drosophila melanogaster. We show that VPO is controlled by a pair of female-specific descending neurons (vpoDNs). The vpoDNs receive excitatory input from auditory neurons (vpoENs), which are tuned to specific features of the D. melanogaster song, and from pC1 neurons, which encode the mating status of the female. The song responses of vpoDNs, but not vpoENs, are attenuated upon mating, accounting for the reduced receptivity of mated females. This modulation is mediated by pC1 neurons. The vpoDNs thus directly integrate the external and internal signals that control the mating decisions of Drosophila females.
The mating decisions of Drosophila melanogaster females are primarily revealed through either of two discrete actions: opening of the vaginal plates to allow copulation, or extrusion of the ovipositor to reject the male. Both actions are triggered by the male courtship song, and both are dependent upon the female's mating status. Virgin females are more likely to open their vaginal plates in response to song; mated females are more likely to extrude their ovipositor. Here, we examine the neural cause and behavioral consequence of ovipositor extrusion. We show that the DNp13 descending neurons act as command-type neurons for ovipositor extrusion, and that ovipositor extrusion is an effective deterrent only when performed by females that have previously mated. The DNp13 neurons respond to male song via direct synaptic input from the pC2l auditory neurons. Mating status does not modulate the song responses of DNp13 neurons, but rather how effectively they can engage the motor circuits for ovipositor extrusion. We present evidence that mating status information is mediated by ppk sensory neurons in the uterus, which are activated upon ovulation. Vaginal plate opening and ovipositor extrusion are thus controlled by anatomically and functionally distinct circuits, highlighting the diversity of neural decision-making circuits even in the context of closely related behaviors with shared exteroceptive and interoceptive inputs.
Mating and egg laying are tightly cooordinated events in the reproductive life of all oviparous females. Oviposition is typically rare in virgin females but is initiated after copulation. Here we identify the neural circuitry that links egg laying to mating status in Drosophila melanogaster. Activation of female-specific oviposition descending neurons (oviDNs) is necessary and sufficient for egg laying, and is equally potent in virgin and mated females. After mating, sex peptide-a protein from the male seminal fluid-triggers many behavioural and physiological changes in the female, including the onset of egg laying. Sex peptide is detected by sensory neurons in the uterus, and silences these neurons and their postsynaptic ascending neurons in the abdominal ganglion. We show that these abdominal ganglion neurons directly activate the female-specific pC1 neurons. GABAergic (γ-aminobutyric-acid-releasing) oviposition inhibitory neurons (oviINs) mediate feed-forward inhibition from pC1 neurons to both oviDNs and their major excitatory input, the oviposition excitatory neurons (oviENs). By attenuating the abdominal ganglion inputs to pC1 neurons and oviINs, sex peptide disinhibits oviDNs to enable egg laying after mating. This circuitry thus coordinates the two key events in female reproduction: mating and egg laying.
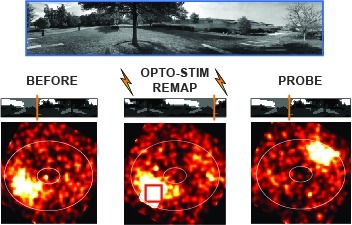
Many animals rely on an internal heading representation when navigating in varied environments. How this representation is linked to the sensory cues that define different surroundings is unclear. In the fly brain, heading is represented by 'compass' neurons that innervate a ring-shaped structure known as the ellipsoid body. Each compass neuron receives inputs from 'ring' neurons that are selective for particular visual features; this combination provides an ideal substrate for the extraction of directional information from a visual scene. Here we combine two-photon calcium imaging and optogenetics in tethered flying flies with circuit modelling, and show how the correlated activity of compass and visual neurons drives plasticity, which flexibly transforms two-dimensional visual cues into a stable heading representation. We also describe how this plasticity enables the fly to convert a partial heading representation, established from orienting within part of a novel setting, into a complete heading representation. Our results provide mechanistic insight into the memory-related computations that are essential for flexible navigation in varied surroundings.