Filter
Associated Lab
- Ahrens Lab (5) Apply Ahrens Lab filter
- Aso Lab (1) Apply Aso Lab filter
- Baker Lab (2) Apply Baker Lab filter
- Betzig Lab (4) Apply Betzig Lab filter
- Bock Lab (2) Apply Bock Lab filter
- Cardona Lab (1) Apply Cardona Lab filter
- Cui Lab (2) Apply Cui Lab filter
- Dickson Lab (3) Apply Dickson Lab filter
- Druckmann Lab (1) Apply Druckmann Lab filter
- Dudman Lab (2) Apply Dudman Lab filter
- Eddy/Rivas Lab (2) Apply Eddy/Rivas Lab filter
- Egnor Lab (1) Apply Egnor Lab filter
- Fetter Lab (3) Apply Fetter Lab filter
- Fitzgerald Lab (1) Apply Fitzgerald Lab filter
- Gonen Lab (11) Apply Gonen Lab filter
- Grigorieff Lab (4) Apply Grigorieff Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Heberlein Lab (6) Apply Heberlein Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Hess Lab (2) Apply Hess Lab filter
- Jayaraman Lab (3) Apply Jayaraman Lab filter
- Ji Lab (1) Apply Ji Lab filter
- Johnson Lab (1) Apply Johnson Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Keller Lab (10) Apply Keller Lab filter
- Lavis Lab (4) Apply Lavis Lab filter
- Leonardo Lab (3) Apply Leonardo Lab filter
- Lippincott-Schwartz Lab (11) Apply Lippincott-Schwartz Lab filter
- Looger Lab (10) Apply Looger Lab filter
- Magee Lab (3) Apply Magee Lab filter
- Menon Lab (3) Apply Menon Lab filter
- Pachitariu Lab (3) Apply Pachitariu Lab filter
- Pavlopoulos Lab (1) Apply Pavlopoulos Lab filter
- Reiser Lab (2) Apply Reiser Lab filter
- Riddiford Lab (5) Apply Riddiford Lab filter
- Romani Lab (1) Apply Romani Lab filter
- Rubin Lab (5) Apply Rubin Lab filter
- Satou Lab (2) Apply Satou Lab filter
- Scheffer Lab (3) Apply Scheffer Lab filter
- Schreiter Lab (7) Apply Schreiter Lab filter
- Sgro Lab (1) Apply Sgro Lab filter
- Singer Lab (9) Apply Singer Lab filter
- Spruston Lab (2) Apply Spruston Lab filter
- Stern Lab (6) Apply Stern Lab filter
- Sternson Lab (3) Apply Sternson Lab filter
- Svoboda Lab (10) Apply Svoboda Lab filter
- Tjian Lab (1) Apply Tjian Lab filter
- Truman Lab (3) Apply Truman Lab filter
- Turaga Lab (2) Apply Turaga Lab filter
- Turner Lab (2) Apply Turner Lab filter
- Wu Lab (3) Apply Wu Lab filter
- Zlatic Lab (2) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- December 2013 (13) Apply December 2013 filter
- November 2013 (10) Apply November 2013 filter
- October 2013 (20) Apply October 2013 filter
- September 2013 (19) Apply September 2013 filter
- August 2013 (15) Apply August 2013 filter
- July 2013 (19) Apply July 2013 filter
- June 2013 (17) Apply June 2013 filter
- May 2013 (10) Apply May 2013 filter
- April 2013 (12) Apply April 2013 filter
- March 2013 (11) Apply March 2013 filter
- February 2013 (19) Apply February 2013 filter
- January 2013 (29) Apply January 2013 filter
- Remove 2013 filter 2013
Type of Publication
194 Publications
Showing 71-80 of 194 resultsMembrane proteins play a tremendously important role in cell physiology and serve as a target for an increasing number of drugs. Structural information is key to understanding their function and for developing new strategies for combating disease. However, the complex physical chemistry associated with membrane proteins has made them more difficult to study than their soluble cousins. Electron crystallography has historically been a successful method for solving membrane protein structures and has the advantage of providing a native lipid environment for these proteins. Specifically, when membrane proteins form two-dimensional arrays within a lipid bilayer, electron microscopy can be used to collect images and diffraction and the corresponding data can be combined to produce a three-dimensional reconstruction, which under favorable conditions can extend to atomic resolution. Like X-ray crystallography, the quality of the structures are very much dependent on the order and size of the crystals. However, unlike X-ray crystallography, high-throughput methods for screening crystallization trials for electron crystallography are not in general use. In this chapter, we describe two alternative methods for high-throughput screening of membrane protein crystallization within the lipid bilayer. The first method relies on the conventional use of dialysis for removing detergent and thus reconstituting the bilayer; an array of dialysis wells in the standard 96-well format allows the use of a liquid-handling robot and greatly increases throughput. The second method relies on titration of cyclodextrin as a chelating agent for detergent; a specialized pipetting robot has been designed not only to add cyclodextrin in a systematic way, but to use light scattering to monitor the reconstitution process. In addition, the use of liquid-handling robots for making negatively stained grids and methods for automatically imaging samples in the electron microscope are described.
All organisms react to noxious and mechanical stimuli but we still lack a complete understanding of cellular and molecular mechanisms by which somatosensory information is transformed into appropriate motor outputs. The small number of neurons and excellent genetic tools make Drosophila larva an especially tractable model system in which to address this problem. We developed high throughput assays with which we can simultaneously expose more than 1,000 larvae per man-hour to precisely timed noxious heat, vibration, air current, or optogenetic stimuli. Using this hardware in combination with custom software we characterized larval reactions to somatosensory stimuli in far greater detail than possible previously. Each stimulus evoked a distinctive escape strategy that consisted of multiple actions. The escape strategy was context-dependent. Using our system we confirmed that the nociceptive class IV multidendritic neurons were involved in the reactions to noxious heat. Chordotonal (ch) neurons were necessary for normal modulation of head casting, crawling and hunching, in response to mechanical stimuli. Consistent with this we observed increases in calcium transients in response to vibration in ch neurons. Optogenetic activation of ch neurons was sufficient to evoke head casting and crawling. These studies significantly increase our understanding of the functional roles of larval ch neurons. More generally, our system and the detailed description of wild type reactions to somatosensory stimuli provide a basis for systematic identification of neurons and genes underlying these behaviors.
During locomotion in vertebrates, reticulospinal neurons in the hindbrain play critical roles in providing descending excitation to the spinal cord locomotor systems. However, despite the fact that many genes that are used to classify the neuronal identities of neurons in the hindbrain have been identified, the molecular identity of the reticulospinal neurons that are critically involved in locomotor drive is not well understood. Chx10-expressing neurons (V2a neurons) are ipsilaterally projecting glutamatergic neurons in the spinal cord and the hindbrain. Many of the V2a neurons in the hindbrain are known to project to the spinal cord in zebrafish, making hindbrain V2a neurons a prime candidate in descending locomotor drive. Results We investigated the roles of hindbrain V2a neurons using optogenetic and electrophysiological approaches. The forced activation of hindbrain V2a neurons using channelrhodopsin efficiently evoked swimming, whereas the forced inactivation of them using Archearhodopsin3 or Halorhodpsin reliably stopped ongoing swimming. Electrophysiological recordings of two populations of hindbrain reticulospinal V2a neurons showed that they were active during swimming. One population of neurons, small V2a neurons in the caudal hindbrain, fired with low rhythmicity, whereas the other population of neurons, large reticulospinal V2a neurons, called MiV1 neurons, fired more rhythmically. Conclusions These results indicated that hindbrain reticulospinal V2a neurons play critical roles in providing excitation to the spinal locomotor circuits during swimming by providing both tonic and phasic inputs to the circuits.
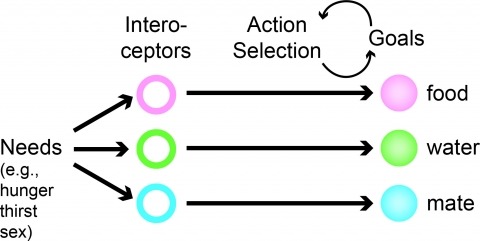
Neural processes that direct an animal’s actions toward environmental goals are critical elements for understanding behavior. The hypothalamus is closely associated with motivated behaviors required for survival and reproduction. Intense feeding, drinking, aggressive, and sexual behaviors can be produced by a simple neuronal stimulus applied to discrete hypothalamic regions. What can these "evoked behaviors" teach us about the neural processes that determine behavioral intent and intensity? Small populations of neurons sufficient to evoke a complex motivated behavior may be used as entry points to identify circuits that energize and direct behavior to specific goals. Here, I review recent applications of molecular genetic, optogenetic, and pharmacogenetic approaches that overcome previous limitations for analyzing anatomically complex hypothalamic circuits and their interactions with the rest of the brain. These new tools have the potential to bridge the gaps between neurobiological and psychological thinking about the mechanisms of complex motivated behavior.
Nonvisual photosensation enables animals to sense light without sight. However, the cellular and molecular mechanisms of nonvisual photobehaviors are poorly understood, especially in vertebrate animals. Here, we describe the photomotor response (PMR), a robust and reproducible series of motor behaviors in zebrafish that is elicited by visual wavelengths of light but does not require the eyes, pineal gland, or other canonical deep-brain photoreceptive organs. Unlike the relatively slow effects of canonical nonvisual pathways, motor circuits are strongly and quickly (seconds) recruited during the PMR behavior. We find that the hindbrain is both necessary and sufficient to drive these behaviors. Using in vivo calcium imaging, we identify a discrete set of neurons within the hindbrain whose responses to light mirror the PMR behavior. Pharmacological inhibition of the visual cycle blocks PMR behaviors, suggesting that opsin-based photoreceptors control this behavior. These data represent the first known light-sensing circuit in the vertebrate hindbrain.
The brain represents sensory information in the coordinated activity of neuronal ensembles. Although the microcircuits underlying olfactory processing are well characterized in Drosophila, no studies to date have examined the encoding of odor identity by populations of neurons and related it to the odor specificity of olfactory behavior. Here we used two-photon Ca(2+) imaging to record odor-evoked responses from >100 neurons simultaneously in the Drosophila mushroom body (MB). For the first time, we demonstrate quantitatively that MB population responses contain substantial information on odor identity. Using a series of increasingly similar odor blends, we identified conditions in which odor discrimination is difficult behaviorally. We found that MB ensemble responses accounted well for olfactory acuity in this task. Kenyon cell ensembles with as few as 25 cells were sufficient to match behavioral discrimination accuracy. Using a generalization task, we demonstrated that the MB population code could predict the flies' responses to novel odors. The degree to which flies generalized a learned aversive association to unfamiliar test odors depended upon the relative similarity between the odors' evoked MB activity patterns. Discrimination and generalization place different demands on the animal, yet the flies' choices in these tasks were reliably predicted based on the amount of overlap between MB activity patterns. Therefore, these different behaviors can be understood in the context of a single physiological framework.
Morphogenesis, the development of the shape of an organism, is a dynamic process on a multitude of scales, from fast subcellular rearrangements and cell movements to slow structural changes at the whole-organism level. Live-imaging approaches based on light microscopy reveal the intricate dynamics of this process and are thus indispensable for investigating the underlying mechanisms. This Review discusses emerging imaging techniques that can record morphogenesis at temporal scales from seconds to days and at spatial scales from hundreds of nanometers to several millimeters. To unlock their full potential, these methods need to be matched with new computational approaches and physical models that help convert highly complex image data sets into biological insights.
Understanding the neural correlates of behavior in the mammalian cortex requires measurements of activity in awake, behaving animals. Rodents have emerged as a powerful model for dissecting the cortical circuits underlying behavior attributable to the convergence of several methods. Genetically encoded calcium indicators combined with viral-mediated or transgenic tools enable chronic monitoring of calcium signals in neuronal populations and subcellular structures of identified cell types. Stable one- and two-photon imaging of neuronal activity in awake, behaving animals is now possible using new behavioral paradigms in head-fixed animals, or using novel miniature head-mounted microscopes in freely moving animals. This mini-symposium will highlight recent applications of these methods for studying sensorimotor integration, decision making, learning, and memory in cortical and subcortical brain areas. We will outline future prospects and challenges for identifying the neural underpinnings of task-dependent behavior using cellular imaging in rodents.
The zebrafish Danio rerio has emerged as a powerful vertebrate model system that lends itself particularly well to quantitative investigations with live imaging approaches, owing to its exceptionally high optical clarity in embryonic and larval stages. Recent advances in light microscopy technology enable comprehensive analyses of cellular dynamics during zebrafish embryonic development, systematic mapping of gene expression dynamics, quantitative reconstruction of mutant phenotypes and the system-level biophysical study of morphogenesis. Despite these technical breakthroughs, it remains challenging to design and implement experiments for in vivo long-term imaging at high spatio-temporal resolution. This article discusses the fundamental challenges in zebrafish long-term live imaging, provides experimental protocols and highlights key properties and capabilities of advanced fluorescence microscopes. The article focuses in particular on experimental assays based on light sheet-based fluorescence microscopy, an emerging imaging technology that achieves exceptionally high imaging speeds and excellent signal-to-noise ratios, while minimizing light-induced damage to the specimen. This unique combination of capabilities makes light sheet microscopy an indispensable tool for the in vivo long-term imaging of large developing organisms.