Filter
Associated Lab
- Ahrens Lab (2) Apply Ahrens Lab filter
- Baker Lab (1) Apply Baker Lab filter
- Druckmann Lab (3) Apply Druckmann Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Hermundstad Lab (7) Apply Hermundstad Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Remove Jayaraman Lab filter Jayaraman Lab
- Ji Lab (1) Apply Ji Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Looger Lab (10) Apply Looger Lab filter
- Podgorski Lab (1) Apply Podgorski Lab filter
- Reiser Lab (2) Apply Reiser Lab filter
- Romani Lab (4) Apply Romani Lab filter
- Rubin Lab (5) Apply Rubin Lab filter
- Saalfeld Lab (1) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Schreiter Lab (9) Apply Schreiter Lab filter
- Svoboda Lab (9) Apply Svoboda Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2024 (1) Apply 2024 filter
- 2023 (1) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (1) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (3) Apply 2018 filter
- 2017 (4) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (4) Apply 2015 filter
- 2014 (1) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (2) Apply 2012 filter
- 2011 (2) Apply 2011 filter
- 2010 (2) Apply 2010 filter
- 2009 (1) Apply 2009 filter
Type of Publication
- Remove Janelia filter Janelia
39 Publications
Showing 11-20 of 39 resultsAnimals use acoustic signals across a variety of social behaviors, particularly courtship. In Drosophila, song is detected by antennal mechanosensory neurons and further processed by second-order aPN1/aLN(al) neurons. However, little is known about the central pathways mediating courtship hearing. In this study, we identified a male-specific pathway for courtship hearing via third-order ventrolateral protocerebrum Projection Neuron 1 (vPN1) neurons and fourth-order pC1 neurons. Genetic inactivation of vPN1 or pC1 disrupts song-induced male-chaining behavior. Calcium imaging reveals that vPN1 responds preferentially to pulse song with long inter-pulse intervals (IPIs), while pC1 responses to pulse song closely match the behavioral chaining responses at different IPIs. Moreover, genetic activation of either vPN1 or pC1 induced courtship chaining, mimicking the behavioral response to song. These results outline the aPN1-vPN1-pC1 pathway as a labeled line for the processing and transformation of courtship song in males.
Seizures induced by visual stimulation (photosensitive epilepsy; PSE) represent a common type of epilepsy in humans, but the molecular mechanisms and genetic drivers underlying PSE remain unknown, and no good genetic animal models have been identified as yet. Here, we show an animal model of PSE, in , owing to defective cortex glia. The cortex glial membranes are severely compromised in ceramide phosphoethanolamine synthase ()-null mutants and fail to encapsulate the neuronal cell bodies in the neuronal cortex. Expression of human sphingomyelin synthase 1, which synthesizes the closely related ceramide phosphocholine (sphingomyelin), rescues the cortex glial abnormalities and PSE, underscoring the evolutionarily conserved role of these lipids in glial membranes. Further, we show the compromise in plasma membrane structure that underlies the glial cell membrane collapse in mutants and leads to the PSE phenotype.
Behavioral strategies employed for chemotaxis have been described across phyla, but the sensorimotor basis of this phenomenon has seldom been studied in naturalistic contexts. Here, we examine how signals experienced during free olfactory behaviors are processed by first-order olfactory sensory neurons (OSNs) of the Drosophila larva. We find that OSNs can act as differentiators that transiently normalize stimulus intensity-a property potentially derived from a combination of integral feedback and feed-forward regulation of olfactory transduction. In olfactory virtual reality experiments, we report that high activity levels of the OSN suppress turning, whereas low activity levels facilitate turning. Using a generalized linear model, we explain how peripheral encoding of olfactory stimuli modulates the probability of switching from a run to a turn. Our work clarifies the link between computations carried out at the sensory periphery and action selection underlying navigation in odor gradients.
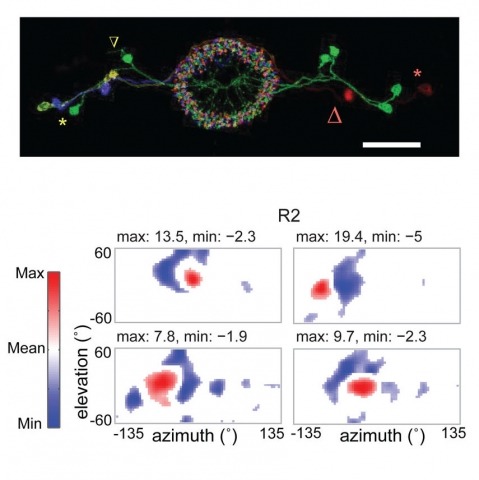
Many animals, including insects, are known to use visual landmarks to orient in their environment. In Drosophila melanogaster, behavioural genetics studies have identified a higher brain structure called the central complex as being required for the fly’s innate responses to vertical visual features and its short- and long-term memory for visual patterns. But whether and how neurons of the fly central complex represent visual features are unknown. Here we use two-photon calcium imaging in head-fixed walking and flying flies to probe visuomotor responses of ring neurons—a class of central complex neurons that have been implicated in landmark-driven spatial memory in walking flies and memory for visual patterns in tethered flying flies. We show that dendrites of ring neurons are visually responsive and arranged retinotopically. Ring neuron receptive fields comprise both excitatory and inhibitory subfields, resembling those of simple cells in the mammalian primary visual cortex. Ring neurons show strong and, in some cases, direction-selective orientation tuning, with a notable preference for vertically oriented features similar to those that evoke innate responses in flies. Visual responses were diminished during flight, but, in contrast with the hypothesized role of the central complex in the control of locomotion, not modulated during walking. Taken together, these results indicate that ring neurons represent behaviourally relevant visual features in the fly’s environment, enabling downstream central complex circuits to produce appropriate motor commands. More broadly, this study opens the door to mechanistic investigations of circuit computations underlying visually guided action selection in the Drosophila central complex.
Internal representations are thought to support the generation of flexible, long-timescale behavioral patterns in both animals and artificial agents. Here, we present a novel conceptual framework for how Drosophila use their internal representation of head direction to maintain preferred headings in their surroundings, and how they learn to modify these preferences in the presence of selective thermal reinforcement. To develop the framework, we analyzed flies’ behavior in a classical operant visual learning paradigm and found that they use stochastically generated fixations and directed turns to express their heading preferences. Symmetries in the visual scene used in the paradigm allowed us to expose how flies’ probabilistic behavior in this setting is tethered to their head direction representation. We describe how flies’ ability to quickly adapt their behavior to the rules of their environment may rest on a behavioral policy whose parameters are flexible but whose form is genetically encoded in the structure of their circuits. Many of the mechanisms we outline may also be relevant for rapidly adaptive behavior driven by internal representations in other animals, including mammals.
Many animals rely on an internal heading representation when navigating in varied environments. How this representation is linked to the sensory cues that define different surroundings is unclear. In the fly brain, heading is represented by 'compass' neurons that innervate a ring-shaped structure known as the ellipsoid body. Each compass neuron receives inputs from 'ring' neurons that are selective for particular visual features; this combination provides an ideal substrate for the extraction of directional information from a visual scene. Here we combine two-photon calcium imaging and optogenetics in tethered flying flies with circuit modelling, and show how the correlated activity of compass and visual neurons drives plasticity, which flexibly transforms two-dimensional visual cues into a stable heading representation. We also describe how this plasticity enables the fly to convert a partial heading representation, established from orienting within part of a novel setting, into a complete heading representation. Our results provide mechanistic insight into the memory-related computations that are essential for flexible navigation in varied surroundings.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The resulting jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and may allow tracking larger populations of neurons using two-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics, and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and tracking large populations of neurons using 2-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
Genetically encoded calcium indicators (GECIs) can be used to image activity in defined neuronal populations. However, current GECIs produce inferior signals compared to synthetic indicators and recording electrodes, precluding detection of low firing rates. We developed a single-wavelength GCaMP2-based GECI (GCaMP3), with increased baseline fluorescence (3-fold), increased dynamic range (3-fold) and higher affinity for calcium (1.3-fold). We detected GCaMP3 fluorescence changes triggered by single action potentials in pyramidal cell dendrites, with signal-to-noise ratio and photostability substantially better than those of GCaMP2, D3cpVenus and TN-XXL. In Caenorhabditis elegans chemosensory neurons and the Drosophila melanogaster antennal lobe, sensory stimulation-evoked fluorescence responses were significantly enhanced with GCaMP3 (4-6-fold). In somatosensory and motor cortical neurons in the intact mouse, GCaMP3 detected calcium transients with amplitudes linearly dependent on action potential number. Long-term imaging in the motor cortex of behaving mice revealed large fluorescence changes in imaged neurons over months.
Optogenetic tools enable examination of how specific cell types contribute to brain circuit functions. A long-standing question is whether it is possible to independently activate two distinct neural populations in mammalian brain tissue. Such a capability would enable the study of how different synapses or pathways interact to encode information in the brain. Here we describe two channelrhodopsins, Chronos and Chrimson, discovered through sequencing and physiological characterization of opsins from over 100 species of alga. Chrimson’s excitation spectrum is red shifted by 45 nm relative to previous channelrhodopsins and can enable experiments in which red light is preferred. We show minimal visual system-mediated behavioral interference when using Chrimson in neurobehavioral studies in Drosophila melanogaster. Chronos has faster kinetics than previous channelrhodopsins yet is effectively more light sensitive. Together these two reagents enable two-color activation of neural spiking and downstream synaptic transmission in independent neural populations without detectable cross-talk in mouse brain slice.