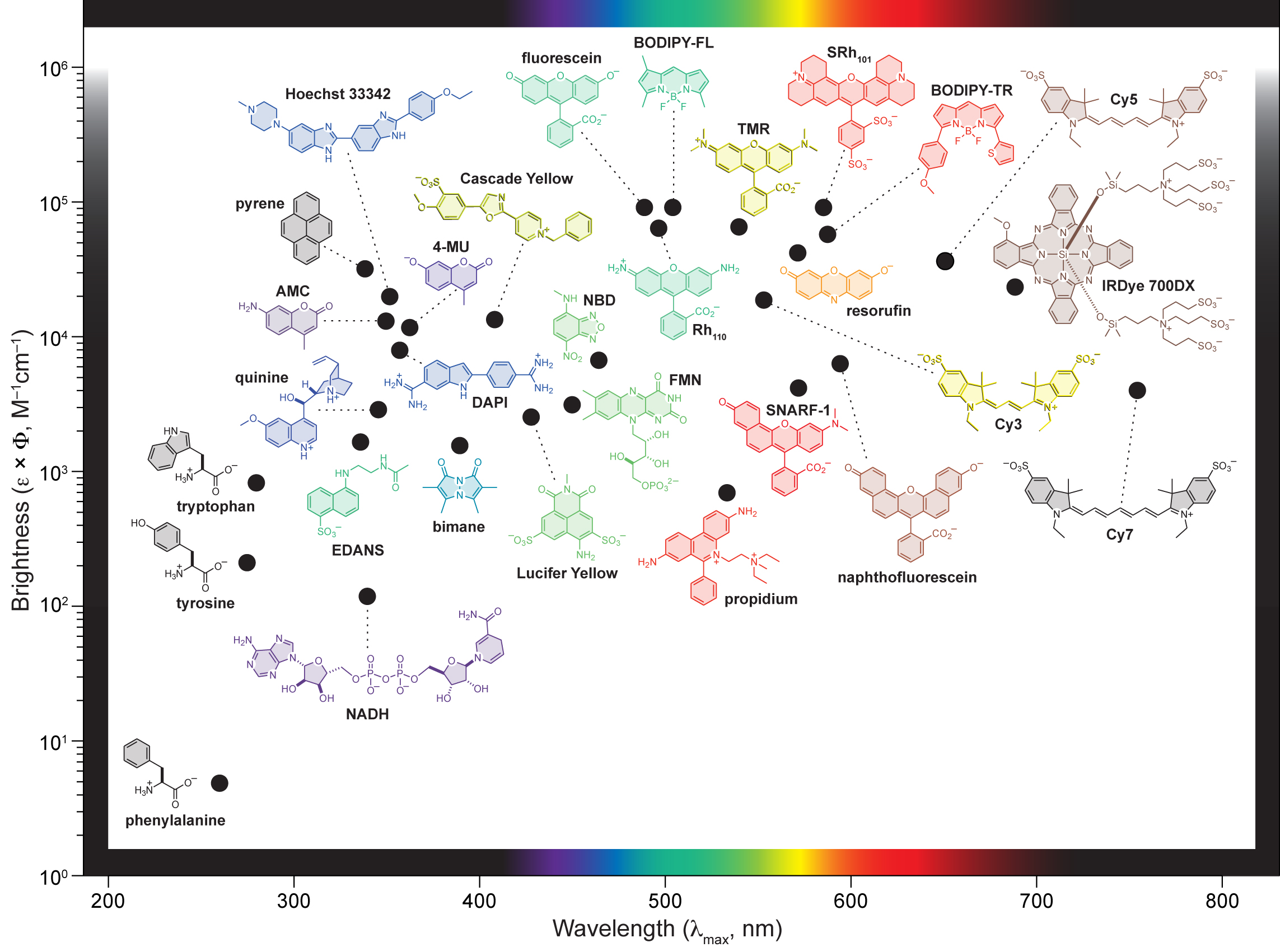
Brighter Dyes
Many of the small molecule fluorophore scaffolds were first discovered over a century ago. This antiquity is a testament to the fantastic chemical and spectroscopic properties of these molecules—they have been distilled by history. From a synthetic chemistry perspective, however, the age of these dyes often means the chemistry we use to build these molecules is also old. Rather than remain complacent about the current portfolio of chemical transformations used in dye research, we explore new synthesis techniques and apply them to the creation of novel dye derivatives. In particular, we have developed new divergent synthetic methodologies using chemical reactions that are typically reserved for drug discovery, allowing the efficient construction of fluorescent dyes. This effort has resulted in the discovery of a new auxochrome for small molecule fluorophores, azetidine, which gives substantial increases in brightness and photostability through a diminutive change in structure. These "Janelia Fluor" dyes enable new and improved single-molecule imaging experiments and we work with the labs of Robert Tjian, Eric Betzig, and James Liu, along with the Transcription Imaging Consortium (TIC) and the Applied Physics and Instrumentation Group (APIG), to push the boundaries of fluorescence microscopy.
Photoactivatable Fluorophores
Light can provide exquisite spatiotemporal control over chemical structure. Photochemical activation of small-molecule agonists, fluorescent dyes, and fluorescent proteins can be used in sophisticated experiments to deliver biologically active molecules or facilitate advanced imaging experiments. Photoactivatable “caged” small-molecule fluorophores have certain advantages over photoactivatable fluorescent proteins including smaller size, faster diffusion times, higher brightness, and an expanded color palette. Despite these advantages, the utility of caged small molecules has languished due to the difficult organic chemistry required to synthesize these compounds. We have developed efficient, general syntheses of highly soluble caged rhodamine and fluorescein dyes to supplement (or supplant) the proteinous caged probes. We collaborate with Eric Betzig and Harald Hess to apply these compounds to the Nobel Prize winning idea of photoactivated localization microscopy (PALM). This technique involves iterative activation and measurement of caged fluorophores within a sample, allowing construction of an "super-resolution" image. We also collaborate with Josh Dudman to develop caged fluorophores for neural tracing experiments and to mark highly active subcellular regions.
Enzyme-Activated Fluorophores
In addition to light, enzymatic activity can be used to activate fluorescence. We can use fluorogenic enzyme substrates to examine the specificity of endogenous esterases in vitro or in live cells to better understand the activity of these enzymes and develop improved delivery strategies for small molecules. In addition, we use fluorogenic compounds to discover and evaluate orthogonal enzyme–substrate pairs for targeted cellular delivery. We work with Loren Looger and Scott Sternson to find enzyme–substrate pairs that allow pharmacological agents to be targeted to genetically defined cellular populations in complex biological environments.
Outlook
The opportunity to interact closely with physicists, engineers, and biologists at Janelia Research Campus provides a fertile place for applying organic chemistry to biological problems. The unique power of chemistry is the capacity to construct molecules of definite structure. As chemists at Janelia, we ply our craft in concert with scientists from other disciplines to shed light on complex problems in neurobiology and beyond.
