Filter
Associated Lab
- Dudman Lab (1) Apply Dudman Lab filter
- Harris Lab (7) Apply Harris Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Lee (Albert) Lab (29) Apply Lee (Albert) Lab filter
- Pachitariu Lab (2) Apply Pachitariu Lab filter
- Romani Lab (1) Apply Romani Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Sternson Lab (1) Apply Sternson Lab filter
- Svoboda Lab (3) Apply Svoboda Lab filter
Publication Date
Type of Publication
- Remove Janelia filter Janelia
29 Publications
Showing 21-29 of 29 resultsIntracellular recording is an essential technique for investigating cellular mechanisms underlying complex brain functions. Despite the high sensitivity of the technique to mechanical disturbances, intracellular recording has been applied to awake, behaving, and even freely moving, animals. Here we summarize recent advances in these methods and their application to the measurement and manipulation of membrane potential dynamics for understanding neuronal computations in behaving animals.
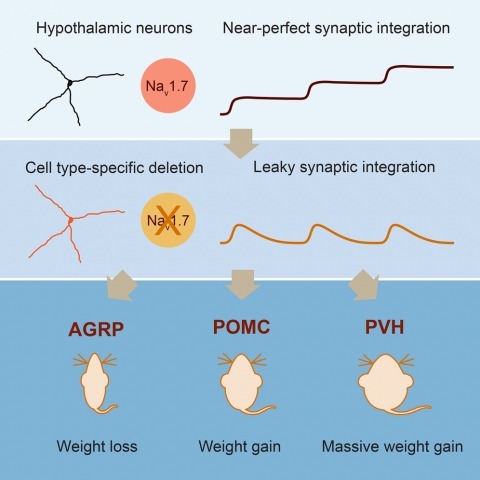
Neurons are well suited for computations on millisecond timescales, but some neuronal circuits set behavioral states over long time periods, such as those involved in energy homeostasis. We found that multiple types of hypothalamic neurons, including those that oppositely regulate body weight, are specialized as near-perfect synaptic integrators that summate inputs over extended timescales. Excitatory postsynaptic potentials (EPSPs) are greatly prolonged, outlasting the neuronal membrane time-constant up to 10-fold. This is due to the voltage-gated sodium channel Nav1.7 (Scn9a), previously associated with pain-sensation but not synaptic integration. Scn9a deletion in AGRP, POMC, or paraventricular hypothalamic neurons reduced EPSP duration, synaptic integration, and altered body weight in mice. In vivo whole-cell recordings in the hypothalamus confirmed near-perfect synaptic integration. These experiments show that integration of synaptic inputs over time by Nav1.7 is critical for body weight regulation and reveal a mechanism for synaptic control of circuits regulating long term homeostatic functions.
Intracellular recording allows precise measurement and manipulation of individual neurons, but it requires stable mechanical contact between the electrode and the cell membrane, and thus it has remained challenging to perform in behaving animals. Whole-cell recordings in freely moving animals can be obtained by rigidly fixing ('anchoring') the pipette electrode to the head; however, previous anchoring procedures were slow and often caused substantial pipette movement, resulting in loss of the recording or of recording quality. We describe a UV-transparent collar and UV-cured adhesive technique that rapidly (within 15 s) anchors pipettes in place with virtually no movement, thus substantially improving the reliability, yield and quality of freely moving whole-cell recordings. Recordings are first obtained from anesthetized or awake head-fixed rats. UV light cures the thin adhesive layers linking pipette to collar to head. Then, the animals are rapidly and smoothly released for recording during unrestrained behavior. The anesthetized-patched version can be completed in ∼4-7 h (excluding histology) and the awake-patched version requires ∼1-4 h per day for ∼2 weeks. These advances should greatly facilitate studies of neuronal integration and plasticity in identified cells during natural behaviors.
The rules governing the formation of spatial maps in the hippocampus have not been determined. We investigated the large-scale structure of place field activity by recording hippocampal neurons in rats exploring a previously unencountered 48-meter-long track. Single-cell and population activities were well described by a two-parameter stochastic model. Individual neurons had their own characteristic propensity for forming fields randomly along the track, with some cells expressing many fields and many exhibiting few or none. Because of the particular distribution of propensities across cells, the number of neurons with fields scaled logarithmically with track length over a wide, ethological range. These features constrain hippocampal memory mechanisms, may allow efficient encoding of environments and experiences of vastly different extents and durations, and could reflect general principles of population coding.
During many natural behaviors the relevant sensory stimuli and motor outputs are difficult to quantify. Furthermore, the high dimensionality of the space of possible stimuli and movements compounds the problem of experimental control. Head fixation facilitates stimulus control and movement tracking, and can be combined with techniques for recording and manipulating neural activity. However, head-fixed mouse behaviors are typically trained through extensive instrumental conditioning. Here we present a whisker-based, tactile virtual reality system for head-fixed mice running on a spherical treadmill. Head-fixed mice displayed natural movements, including running and rhythmic whisking at 16 Hz. Whisking was centered on a set point that changed in concert with running so that more protracted whisking was correlated with faster running. During turning, whiskers moved in an asymmetric manner, with more retracted whisker positions in the turn direction and protracted whisker movements on the other side. Under some conditions, whisker movements were phase-coupled to strides. We simulated a virtual reality tactile corridor, consisting of two moveable walls controlled in a closed-loop by running speed and direction. Mice used their whiskers to track the walls of the winding corridor without training. Whisker curvature changes, which cause forces in the sensory follicles at the base of the whiskers, were tightly coupled to distance from the walls. Our behavioral system allows for precise control of sensorimotor variables during natural tactile navigation.
The patch-clamp technique and the whole-cell measurements derived from it have greatly advanced our understanding of the coding properties of individual neurons by allowing for a detailed analysis of their excitatory/inhibitory synaptic inputs, intrinsic electrical properties, and morphology. Because such measurements require a high level of mechanical stability they have for a long time been limited to in vitro and anesthetized preparations. Recently, however, a considerable amount of effort has been devoted to extending these techniques to awake restrained/head-fixed preparations allowing for the study of the input-output functions of neurons during behavior. In this chapter we describe a technique extending patch-clamp recordings to awake animals free to explore their environments.
The origin of the spatial receptive fields of hippocampal place cells has not been established. A hippocampal CA1 pyramidal cell receives thousands of synaptic inputs, mostly from other spatially tuned neurons; however, how the postsynaptic neuron’s cellular properties determine the response to these inputs during behavior is unknown. We discovered that, contrary to expectations from basic models of place cells and neuronal integration, a small, spatially uniform depolarization of the spatially untuned somatic membrane potential of a silent cell leads to the sudden and reversible emergence of a spatially tuned subthreshold response and place-field spiking. Such gating of inputs by postsynaptic neuronal excitability reveals a cellular mechanism for receptive field origin and may be critical for the formation of hippocampal memory representations.
Electrophysiological recordings from behaving animals provide an unparalleled view into the functional role of individual neurons. Intracellular approaches can be especially revealing as they provide information about a neuron's inputs and intrinsic cellular properties, which together determine its spiking output. Recent technical developments have made intracellular recording possible during an ever-increasing range of behaviors in both head-fixed and freely moving animals. These recordings have yielded fundamental insights into the cellular and circuit mechanisms underlying neural activity during natural behaviors in such areas as sensory perception, motor sequence generation, and spatial navigation, forging a direct link between cellular and systems neuroscience.
For each environment a rodent has explored, its hippocampus contains a map consisting of a unique subset of neurons, called place cells, that have spatially tuned spiking there, with the remaining neurons being essentially silent. Using whole-cell recording in freely moving rats exploring a novel maze, we observed differences in intrinsic cellular properties and input-based subthreshold membrane potential levels underlying this division into place and silent cells. Compared to silent cells, place cells had lower spike thresholds and peaked versus flat subthreshold membrane potentials as a function of animal location. Both differences were evident from the beginning of exploration. Additionally, future place cells exhibited higher burst propensity before exploration. Thus, internal settings appear to predetermine which cells will represent the next novel environment encountered. Furthermore, place cells fired spatially tuned bursts with large, putatively calcium-mediated depolarizations that could trigger plasticity and stabilize the new map for long-term storage. Our results provide new insight into hippocampal memory formation.