Filter
Associated Lab
- Dudman Lab (1) Apply Dudman Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Lavis Lab (1) Apply Lavis Lab filter
- Lee (Albert) Lab (1) Apply Lee (Albert) Lab filter
- Looger Lab (2) Apply Looger Lab filter
- Pachitariu Lab (1) Apply Pachitariu Lab filter
- Saalfeld Lab (2) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Remove Sternson Lab filter Sternson Lab
- Svoboda Lab (4) Apply Svoboda Lab filter
- Tillberg Lab (3) Apply Tillberg Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
Associated Project Team
Associated Support Team
Publication Date
- 2023 (2) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (3) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (2) Apply 2019 filter
- 2017 (2) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (7) Apply 2015 filter
- 2014 (5) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (4) Apply 2012 filter
- 2011 (6) Apply 2011 filter
- 2010 (1) Apply 2010 filter
- 2009 (1) Apply 2009 filter
- 2008 (1) Apply 2008 filter
47 Janelia Publications
Showing 31-40 of 47 resultsA specialist neuron uses an intriguing process to help control the body's response to hunger. A lipid pathway involving the breakdown of cellular components regulates the expression of a neuropeptide that affects feeding and body weight.
Primary aldosteronism (PA) is the most frequent form of secondary hypertension. Over the past two decades, major advances have been made in our understanding of the disease with the identification of germline or somatic mutations in ion channels and pumps. These mutations enhance calcium signaling, the main trigger of aldosterone biosynthesis.
The central nucleus of the amygdala (CEA) is a brain region that integrates external and internal sensory information and executes innate and adaptive behaviors through distinct output pathways. Despite its complex functions, the diversity of molecularly defined neuronal types in the CEA and their contributions to major axonal projection targets have not been examined systematically. Here, we performed single-cell RNA-sequencing (scRNA-Seq) to classify molecularly defined cell types in the CEA and identified marker-genes to map the location of these neuronal types using expansion assisted iterative fluorescence in situ hybridization (EASI-FISH). We developed new methods to integrate EASI-FISH with 5-plex retrograde axonal labeling to determine the spatial, morphological, and connectivity properties of ∼30,000 molecularly defined CEA neurons. Our study revealed spatio-molecular organization of the CEA, with medial and lateral CEA associated with distinct cell families. We also found a long-range axon projection network from the CEA, where target regions receive inputs from multiple molecularly defined cell types. Axon collateralization was found primarily among projections to hindbrain targets, which are distinct from forebrain projections. This resource reports marker-gene combinations for molecularly defined cell types and axon-projection types, which will be useful for selective interrogation of these neuronal populations to study their contributions to the diverse functions of the CEA.
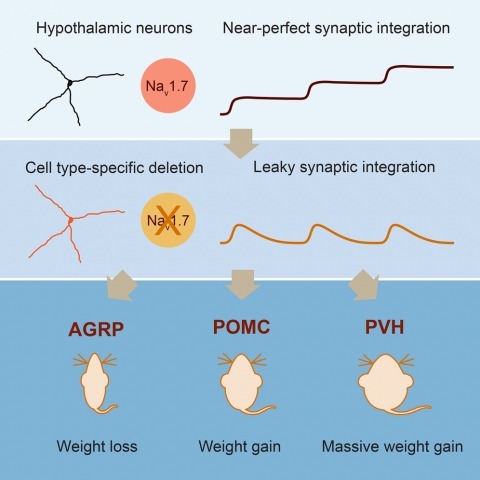
Neurons are well suited for computations on millisecond timescales, but some neuronal circuits set behavioral states over long time periods, such as those involved in energy homeostasis. We found that multiple types of hypothalamic neurons, including those that oppositely regulate body weight, are specialized as near-perfect synaptic integrators that summate inputs over extended timescales. Excitatory postsynaptic potentials (EPSPs) are greatly prolonged, outlasting the neuronal membrane time-constant up to 10-fold. This is due to the voltage-gated sodium channel Nav1.7 (Scn9a), previously associated with pain-sensation but not synaptic integration. Scn9a deletion in AGRP, POMC, or paraventricular hypothalamic neurons reduced EPSP duration, synaptic integration, and altered body weight in mice. In vivo whole-cell recordings in the hypothalamus confirmed near-perfect synaptic integration. These experiments show that integration of synaptic inputs over time by Nav1.7 is critical for body weight regulation and reveal a mechanism for synaptic control of circuits regulating long term homeostatic functions.
How does an organism’s internal state direct its actions? At one moment an animal forages for food with acrobatic feats such as tree climbing and jumping between branches. At another time, it travels along the ground to find water or a mate, exposing itself to predators along the way. These behaviors are costly in terms of energy or physical risk, and the likelihood of performing one set of actions relative to another is strongly modulated by internal state. For example, an animal in energy deficit searches for food and a dehydrated animal looks for water. The crosstalk between physiological state and motivational processes influences behavioral intensity and intent, but the underlying neural circuits are poorly understood. Molecular genetics along with optogenetic and pharmacogenetic tools for perturbing neuron function have enabled cell type-selective dissection of circuits that mediate behavioral responses to physiological state changes. Here, we review recent progress into neural circuit analysis of hunger in the mouse by focusing on a starvation-sensitive neuron population in the hypothalamus that is sufficient to promote voracious eating. We also consider research into the motivational processes that are thought to underlie hunger in order to outline considerations for bridging the gap between homeostatic and motivational neural circuits.
Mice lacking leptin receptors are grossly obese and diabetic, in part due to dysfunction in brain circuits important for energy homeostasis. Transplantation of leptin receptor-expressing hypothalamic progenitor neurons into the brains of leptin receptor deficient mice led to integration into neural circuits, reduced obesity, and normalized circulating glucose levels.
Homeostasis is a biological principle for regulation of essential physiological parameters within a set range. Behavioural responses due to deviation from homeostasis are critical for survival, but motivational processes engaged by physiological need states are incompletely understood. We examined motivational characteristics of two separate neuron populations that regulate energy and fluid homeostasis by using cell-type-specific activity manipulations in mice. We found that starvation-sensitive AGRP neurons exhibit properties consistent with a negative-valence teaching signal. Mice avoided activation of AGRP neurons, indicating that AGRP neuron activity has negative valence. AGRP neuron inhibition conditioned preference for flavours and places. Correspondingly, deep-brain calcium imaging revealed that AGRP neuron activity rapidly reduced in response to food-related cues. Complementary experiments activating thirst-promoting neurons also conditioned avoidance. Therefore, these need-sensing neurons condition preference for environmental cues associated with nutrient or water ingestion, which is learned through reduction of negative-valence signals during restoration of homeostasis.
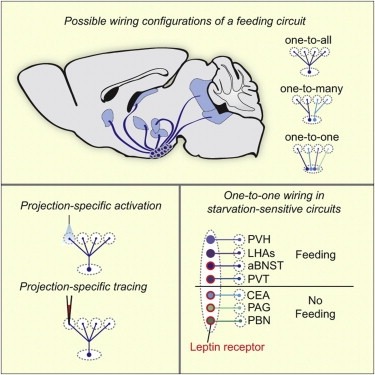
Neural circuits for essential natural behaviors are shaped by selective pressure to coordinate reliable execution of flexible goal-directed actions. However, the structural and functional organization of survival-oriented circuits is poorly understood due to exceptionally complex neuroanatomy. This is exemplified by AGRP neurons, which are a molecularly defined population that is sufficient to rapidly coordinate voracious food seeking and consumption behaviors. Here, we use cell-type-specific techniques for neural circuit manipulation and projection-specific anatomical analysis to examine the organization of this critical homeostatic circuit that regulates feeding. We show that AGRP neuronal circuits use a segregated, parallel, and redundant output configuration. AGRP neuron axon projections that target different brain regions originate from distinct subpopulations, several of which are sufficient to independently evoke feeding. The concerted anatomical and functional analysis of AGRP neuron projection populations reveals a constellation of core forebrain nodes, which are part of an extended circuit that mediates feeding behavior.
The dorsal raphe nucleus (DRN) is an important brain area for body-weight regulation. In this issue of Cell, Nectow et al. uncover cell-type-specific neural circuitry and pharmacology for appetite control within the DRN.