Filter
Associated Lab
- Betzig Lab (1) Apply Betzig Lab filter
- Bock Lab (1) Apply Bock Lab filter
- Eddy/Rivas Lab (1) Apply Eddy/Rivas Lab filter
- Gonen Lab (1) Apply Gonen Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Jayaraman Lab (2) Apply Jayaraman Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Leonardo Lab (1) Apply Leonardo Lab filter
- Looger Lab (1) Apply Looger Lab filter
- Menon Lab (2) Apply Menon Lab filter
- Schreiter Lab (2) Apply Schreiter Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Stern Lab (1) Apply Stern Lab filter
- Svoboda Lab (2) Apply Svoboda Lab filter
Associated Project Team
Associated Support Team
Publication Date
- October 30, 2013 (1) Apply October 30, 2013 filter
- October 29, 2013 (1) Apply October 29, 2013 filter
- October 25, 2013 (1) Apply October 25, 2013 filter
- October 23, 2013 (1) Apply October 23, 2013 filter
- October 19, 2013 (1) Apply October 19, 2013 filter
- October 15, 2013 (1) Apply October 15, 2013 filter
- October 14, 2013 (1) Apply October 14, 2013 filter
- October 11, 2013 (1) Apply October 11, 2013 filter
- October 9, 2013 (1) Apply October 9, 2013 filter
- October 4, 2013 (1) Apply October 4, 2013 filter
- October 1, 2013 (6) Apply October 1, 2013 filter
- Remove October 2013 filter October 2013
- Remove 2013 filter 2013
16 Janelia Publications
Showing 1-10 of 16 resultsFluorescent protein-based sensors for detecting neuronal activity have been developed largely based on non-neuronal screening systems. However, the dynamics of neuronal state variables (e.g., voltage, calcium, etc.) are typically very rapid compared to those of non-excitable cells. We developed an electrical stimulation and fluorescence imaging platform based on dissociated rat primary neuronal cultures. We describe its use in testing genetically-encoded calcium indicators (GECIs). Efficient neuronal GECI expression was achieved using lentiviruses containing a neuronal-selective gene promoter. Action potentials (APs) and thus neuronal calcium levels were quantitatively controlled by electrical field stimulation, and fluorescence images were recorded. Images were segmented to extract fluorescence signals corresponding to individual GECI-expressing neurons, which improved sensitivity over full-field measurements. We demonstrate the superiority of screening GECIs in neurons compared with solution measurements. Neuronal screening was useful for efficient identification of variants with both improved response kinetics and high signal amplitudes. This platform can be used to screen many types of sensors with cellular resolution under realistic conditions where neuronal state variables are in relevant ranges with respect to timing and amplitude.
The lobula giant movement detector (LGMD) is a large-field visual interneuron believed to be involved in collision avoidance and escape behaviors in orthopteran insects, such as locusts. Responses to approaching—or looming—stimuli are highly stereotypical, producing a peak that signals an angular size threshold. Over the past several decades, investigators have elucidated many of the mechanisms underpinning this response, demonstrating that the LGMD implements a multiplication in log-transformed coordinates. Furthermore, the LGMD possesses several mechanisms that preclude it responding to non-looming stimuli. This chapter explores these biophysical mechanisms, as well as highlighting insights the LGMD provides into general principles of dendritic integration.
The ability to localize proteins precisely within subcellular space is crucial to understanding the functioning of biological systems. Recently, we described a protocol that correlates a precise map of fluorescent fusion proteins localized using three-dimensional super-resolution optical microscopy with the fine ultrastructural context of three-dimensional electron micrographs. While it achieved the difficult simultaneous objectives of high photoactivated fluorophore preservation and ultrastructure preservation, it required a super-resolution optical and specialized electron microscope that is not available to many researchers. We present here a faster and more practical protocol with the advantage of a simpler two-dimensional optical (Photoactivated Localization Microscopy (PALM)) and scanning electron microscope (SEM) system that retains the often mutually exclusive attributes of fluorophore preservation and ultrastructure preservation. As before, cryosections were prepared using the Tokuyasu protocol, but the staining protocol was modified to be amenable for use in a standard SEM without the need for focused ion beam ablation. We show the versatility of this technique by labeling different cellular compartments and structures including mitochondrial nucleoids, peroxisomes, and the nuclear lamina. We also demonstrate simultaneous two-color PALM imaging with correlated electron micrographs. Lastly, this technique can be used with small-molecule dyes as demonstrated with actin labeling using phalloidin conjugated to a caged dye. By retaining the dense protein labeling expected for super-resolution microscopy combined with ultrastructural preservation, simplifying the tools required for correlative microscopy, and expanding the number of useful labels we expect this method to be accessible and valuable to a wide variety of researchers.
Mouse visual cortex is subdivided into multiple distinct, hierarchically organized areas that are interconnected through feedforward (FF) and feedback (FB) pathways. The principal synaptic targets of FF and FB axons that reciprocally interconnect primary visual cortex (V1) with the higher lateromedial extrastriate area (LM) are pyramidal cells (Pyr) and parvalbumin (PV)-expressing GABAergic interneurons. Recordings in slices of mouse visual cortex have shown that layer 2/3 Pyr cells receive excitatory monosynaptic FF and FB inputs, which are opposed by disynaptic inhibition. Most notably, inhibition is stronger in the FF than FB pathway, suggesting pathway-specific organization of feedforward inhibition (FFI). To explore the hypothesis that this difference is due to diverse pathway-specific strengths of the inputs to PV neurons we have performed subcellular Channelrhodopsin-2-assisted circuit mapping in slices of mouse visual cortex. Whole-cell patch-clamp recordings were obtained from retrobead-labeled FFV1→LM- and FBLM→V1-projecting Pyr cells, as well as from tdTomato-expressing PV neurons. The results show that the FFV1→LM pathway provides on average 3.7-fold stronger depolarizing input to layer 2/3 inhibitory PV neurons than to neighboring excitatory Pyr cells. In the FBLM→V1 pathway, depolarizing inputs to layer 2/3 PV neurons and Pyr cells were balanced. Balanced inputs were also found in the FFV1→LM pathway to layer 5 PV neurons and Pyr cells, whereas FBLM→V1 inputs to layer 5 were biased toward Pyr cells. The findings indicate that FFI in FFV1→LM and FBLM→V1 circuits are organized in a pathway- and lamina-specific fashion.
Clusters of time series data may change location and memberships over time; in gene expression data, this occurs as groups of genes or samples respond differently to stimuli or experimental conditions at different times. In order to uncover this underlying temporal structure, we consider dynamic clusters with time-dependent parameters which split and merge over time, enabling cluster memberships to change. These interesting time-dependent structures are useful in understanding the development of organisms or complex organs, and could not be identified using traditional clustering methods. In cell cycle data, these time-dependent structure may provide links between genes and stages of the cell cycle, whilst in developmental data sets they may highlight key developmental transitions.
Gap junctions (GJs) represent connexin-rich membrane domains that connect interiors of adjoining cells in mammalian tissues. How fast GJs can respond to bacterial pathogens has not been known previously. Using Bessel beam plane illumination and confocal spinning disk microscopy, we found fast ( 500 ms) formation of connexin-depleted regions (CDRs) inside GJ plaques between cells exposed to AB5 toxins. CDR formation appears as a fast redistribution of connexin channels within GJ plaques with minor changes in outline or geometry. CDR formation does not depend on membrane trafficking or submembrane cytoskeleton and has no effect on GJ conductance. However, CDR responses depend on membrane lipids, can be modified by cholesterol-clustering agents and extracellular K(+) ion concentration, and influence cAMP signaling. The CDR response of GJ plaques to bacterial toxins is a phenomenon observed for all tested connexin isoforms. Through signaling, the CDR response may enable cells to sense exposure to AB5 toxins. CDR formation may reflect lipid-phase separation events in the biological membrane of the GJ plaque, leading to increased connexin packing and lipid reorganization. Our data demonstrate very fast dynamics (in the millisecond-to-second range) within GJ plaques, which previously were considered to be relatively stable, long-lived structures.
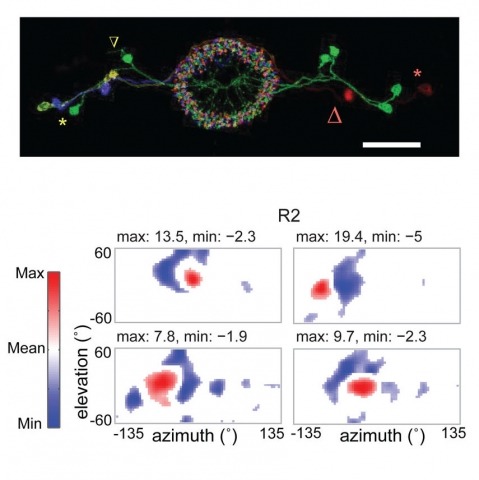
Many animals, including insects, are known to use visual landmarks to orient in their environment. In Drosophila melanogaster, behavioural genetics studies have identified a higher brain structure called the central complex as being required for the fly’s innate responses to vertical visual features and its short- and long-term memory for visual patterns. But whether and how neurons of the fly central complex represent visual features are unknown. Here we use two-photon calcium imaging in head-fixed walking and flying flies to probe visuomotor responses of ring neurons—a class of central complex neurons that have been implicated in landmark-driven spatial memory in walking flies and memory for visual patterns in tethered flying flies. We show that dendrites of ring neurons are visually responsive and arranged retinotopically. Ring neuron receptive fields comprise both excitatory and inhibitory subfields, resembling those of simple cells in the mammalian primary visual cortex. Ring neurons show strong and, in some cases, direction-selective orientation tuning, with a notable preference for vertically oriented features similar to those that evoke innate responses in flies. Visual responses were diminished during flight, but, in contrast with the hypothesized role of the central complex in the control of locomotion, not modulated during walking. Taken together, these results indicate that ring neurons represent behaviourally relevant visual features in the fly’s environment, enabling downstream central complex circuits to produce appropriate motor commands. More broadly, this study opens the door to mechanistic investigations of circuit computations underlying visually guided action selection in the Drosophila central complex.
The second messenger cyclic AMP (cAMP) operates in discrete subcellular regions within which proteins that synthesize, break down or respond to the second messenger are precisely organized. A burgeoning knowledge of compartmentalized cAMP signaling is revealing how the local control of signaling enzyme activity impacts upon disease. The aim of this Cell Science at a Glance article and the accompanying poster is to highlight how misregulation of local cyclic AMP signaling can have pathophysiological consequences. We first introduce the core molecular machinery for cAMP signaling, which includes the cAMP-dependent protein kinase (PKA), and then consider the role of A-kinase anchoring proteins (AKAPs) in coordinating different cAMP-responsive proteins. The latter sections illustrate the emerging role of local cAMP signaling in four disease areas: cataracts, cancer, diabetes and cardiovascular diseases.
Abstract We recently described a new form of neural integration and firing in a subset of interneurons, in which evoking hundreds of action potentials over tens of seconds to minutes produces a sudden barrage of action potentials lasting about a minute beyond the inciting stimulation. During this persistent firing, action potentials are generated in the distal axon and propagate retrogradely to the soma. To distinguish this from other forms of persistent firing, we refer to it here as ’retroaxonal barrage firing’, or ’barrage firing’ for short. Its induction is blocked by chemical inhibitors of gap junctions and curiously, stimulation of one interneuron in some cases triggers barrage firing in a nearby, unstimulated interneuron. Beyond these clues, the mechanisms of barrage firing are unknown. Here we report new results related to these mechanisms. Induction of barrage firing was blocked by lowering extracellular calcium, as long as normal action potential threshold was maintained, and it was inhibited by blocking L-type voltage-gated calcium channels. Despite its calcium dependence, barrage firing was not prevented by inhibiting chemical synaptic transmission. Furthermore, loading the stimulated/recorded interneuron with BAPTA did not block barrage firing, suggesting that the required calcium entry occurs in other cells. Finally, barrage firing was normal in mice with deletion of the primary gene for neuronal gap junctions (connexin36), suggesting that non-neuronal gap junctions may be involved. Together, these findings suggest that barrage firing is probably triggered by a multicellular mechanism involving calcium signalling and gap junctions, but operating independently of chemical synaptic transmission.
SUMMARY: Sequence database searches are an essential part of molecular biology, providing information about the function and evolutionary history of proteins, RNA molecules and DNA sequence elements. We present a tool for DNA/DNA sequence comparison that is built on the HMMER framework, which applies probabilistic inference methods based on hidden Markov models to the problem of homology search. This tool, called nhmmer, enables improved detection of remote DNA homologs, and has been used in combination with Dfam and RepeatMasker to improve annotation of transposable elements in the human genome. AVAILABILITY: nhmmer is a part of the new HMMER3.1 release. Source code and documentation can be downloaded from http://hmmer.org. HMMER3.1 is freely licensed under the GNU GPLv3 and should be portable to any POSIX-compliant operating system, including Linux and Mac OS/X. CONTACT: wheelert@janelia.hhmi.org.