Filter
Associated Lab
4 Janelia Publications
Showing 1-4 of 4 resultsFluorescent proteins and their engineered variants have played an important role in the study of biology. The genetically encoded calcium-indicator protein GCaMP2 comprises a circularly permuted fluorescent protein coupled to the calcium-binding protein calmodulin and a calmodulin target peptide, M13, derived from the intracellular calmodulin target myosin light-chain kinase and has been used to image calcium transients in vivo. To aid rational efforts to engineer improved variants of GCaMP2, this protein was crystallized in the calcium-saturated form. X-ray diffraction data were collected to 2.0 A resolution. The crystals belong to space group C2, with unit-cell parameters a = 126.1.
In neurons, individual dendritic spines isolate N-methyl-d-aspartate (NMDA) receptor-mediated calcium ion (Ca2+) accumulations from the dendrite and other spines. However, the extent to which spines compartmentalize signaling events downstream of Ca2+ influx is not known. We combined two-photon fluorescence lifetime imaging with two-photon glutamate uncaging to image the activity of the small guanosine triphosphatase Ras after NMDA receptor activation at individual spines. Induction of long-term potentiation (LTP) triggered robust Ca2+-dependent Ras activation in single spines that decayed in approximately 5 minutes. Ras activity spread over approximately 10 micrometers of dendrite and invaded neighboring spines by diffusion. The spread of Ras-dependent signaling was necessary for the local regulation of the threshold for LTP induction. Thus, Ca2+-dependent synaptic signals can spread to couple multiple synapses on short stretches of dendrite.
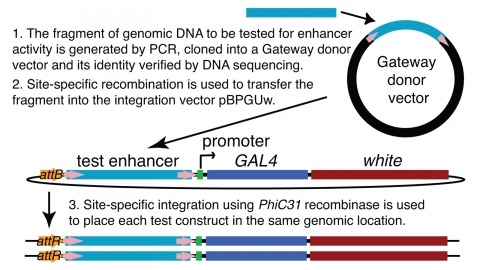
We demonstrate the feasibility of generating thousands of transgenic Drosophila melanogaster lines in which the expression of an exogenous gene is reproducibly directed to distinct small subsets of cells in the adult brain. We expect the expression patterns produced by the collection of 5,000 lines that we are currently generating to encompass all neurons in the brain in a variety of intersecting patterns. Overlapping 3-kb DNA fragments from the flanking noncoding and intronic regions of genes thought to have patterned expression in the adult brain were inserted into a defined genomic location by site-specific recombination. These fragments were then assayed for their ability to function as transcriptional enhancers in conjunction with a synthetic core promoter designed to work with a wide variety of enhancer types. An analysis of 44 fragments from four genes found that >80% drive expression patterns in the brain; the observed patterns were, on average, comprised of <100 cells. Our results suggest that the D. melanogaster genome contains >50,000 enhancers and that multiple enhancers drive distinct subsets of expression of a gene in each tissue and developmental stage. We expect that these lines will be valuable tools for neuroanatomy as well as for the elucidation of neuronal circuits and information flow in the fly brain.