Filter
Associated Lab
- Ahrens Lab (4) Apply Ahrens Lab filter
- Betzig Lab (2) Apply Betzig Lab filter
- Branson Lab (1) Apply Branson Lab filter
- Darshan Lab (3) Apply Darshan Lab filter
- Druckmann Lab (5) Apply Druckmann Lab filter
- Dudman Lab (3) Apply Dudman Lab filter
- Fetter Lab (1) Apply Fetter Lab filter
- Freeman Lab (3) Apply Freeman Lab filter
- Harris Lab (6) Apply Harris Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Jayaraman Lab (9) Apply Jayaraman Lab filter
- Ji Lab (2) Apply Ji Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Lavis Lab (4) Apply Lavis Lab filter
- Lee (Albert) Lab (3) Apply Lee (Albert) Lab filter
- Leonardo Lab (2) Apply Leonardo Lab filter
- Liu (Zhe) Lab (1) Apply Liu (Zhe) Lab filter
- Looger Lab (20) Apply Looger Lab filter
- Magee Lab (1) Apply Magee Lab filter
- Pachitariu Lab (2) Apply Pachitariu Lab filter
- Podgorski Lab (2) Apply Podgorski Lab filter
- Romani Lab (5) Apply Romani Lab filter
- Rubin Lab (3) Apply Rubin Lab filter
- Saalfeld Lab (2) Apply Saalfeld Lab filter
- Schreiter Lab (13) Apply Schreiter Lab filter
- Spruston Lab (3) Apply Spruston Lab filter
- Sternson Lab (4) Apply Sternson Lab filter
- Remove Svoboda Lab filter Svoboda Lab
- Tillberg Lab (3) Apply Tillberg Lab filter
- Turner Lab (3) Apply Turner Lab filter
Associated Project Team
Associated Support Team
- Anatomy and Histology (4) Apply Anatomy and Histology filter
- Gene Targeting and Transgenics (3) Apply Gene Targeting and Transgenics filter
- Integrative Imaging (2) Apply Integrative Imaging filter
- Janelia Experimental Technology (4) Apply Janelia Experimental Technology filter
- Molecular Genomics (2) Apply Molecular Genomics filter
- Primary & iPS Cell Culture (2) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (1) Apply Project Technical Resources filter
- Quantitative Genomics (1) Apply Quantitative Genomics filter
- Scientific Computing Software (2) Apply Scientific Computing Software filter
- Viral Tools (2) Apply Viral Tools filter
Publication Date
- 2023 (5) Apply 2023 filter
- 2022 (6) Apply 2022 filter
- 2021 (7) Apply 2021 filter
- 2020 (5) Apply 2020 filter
- 2019 (14) Apply 2019 filter
- 2018 (11) Apply 2018 filter
- 2017 (9) Apply 2017 filter
- 2016 (8) Apply 2016 filter
- 2015 (9) Apply 2015 filter
- 2014 (7) Apply 2014 filter
- 2013 (10) Apply 2013 filter
- 2012 (9) Apply 2012 filter
- 2011 (7) Apply 2011 filter
- 2010 (7) Apply 2010 filter
- 2009 (8) Apply 2009 filter
- 2008 (6) Apply 2008 filter
- 2007 (3) Apply 2007 filter
131 Janelia Publications
Showing 81-90 of 131 resultsNeurons in motor cortex and connected brain regions fire in anticipation of specific movements, long before movement occurs. This neural activity reflects internal processes by which the brain plans and executes volitional movements. The study of motor planning offers an opportunity to understand how the structure and dynamics of neural circuits support persistent internal states and how these states influence behavior. Recent advances in large-scale neural recordings are beginning to decipher the relationship of the dynamics of populations of neurons during motor planning and movements. New behavioral tasks in rodents, together with quantified perturbations, link dynamics in specific nodes of neural circuits to behavior. These studies reveal a neural network distributed across multiple brain regions that collectively supports motor planning. We review recent advances and highlight areas where further work is needed to achieve a deeper understanding of the mechanisms underlying motor planning and related cognitive processes.
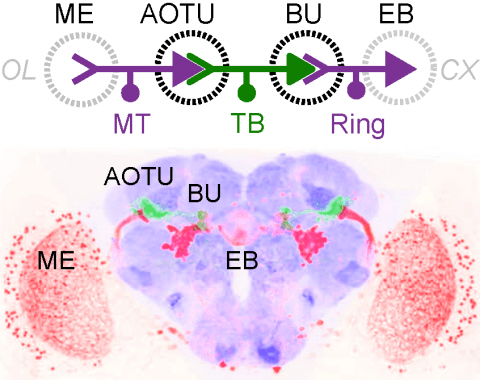
Many animals orient using visual cues, but how a single cue is selected from among many is poorly understood. Here we show that Drosophila ring neurons—central brain neurons implicated in navigation—display visual stimulus selection. Using in vivo two-color two-photon imaging with genetically encoded calcium indicators, we demonstrate that individual ring neurons inherit simple-cell-like receptive fields from their upstream partners. Stimuli in the contralateral visual field suppressed responses to ipsilateral stimuli in both populations. Suppression strength depended on when and where the contralateral stimulus was presented, an effect stronger in ring neurons than in their upstream inputs. This history-dependent effect on the temporal structure of visual responses, which was well modeled by a simple biphasic filter, may determine how visual references are selected for the fly's internal compass. Our approach highlights how two-color calcium imaging can help identify and localize the origins of sensory transformations across synaptically connected neural populations.
The Neurodata Without Borders (NWB) initiative promotes data standardization in neuroscience to increase research reproducibility and opportunities. In the first NWB pilot project, neurophysiologists and software developers produced a common data format for recordings and metadata of cellular electrophysiology and optical imaging experiments. The format specification, application programming interfaces, and sample datasets have been released.
Measuring the dynamics of neural processing across time scales requires following the spiking of thousands of individual neurons over milliseconds and months. To address this need, we introduce the Neuropixels 2.0 probe together with newly designed analysis algorithms. The probe has more than 5000 sites and is miniaturized to facilitate chronic implants in small mammals and recording during unrestrained behavior. High-quality recordings over long time scales were reliably obtained in mice and rats in six laboratories. Improved site density and arrangement combined with newly created data processing methods enable automatic post hoc correction for brain movements, allowing recording from the same neurons for more than 2 months. These probes and algorithms enable stable recordings from thousands of sites during free behavior, even in small animals such as mice.
Active dendrites provide neurons with powerful processing capabilities. However, little is known about the role of neuronal dendrites in behaviourally related circuit computations. Here we report that a novel global dendritic nonlinearity is involved in the integration of sensory and motor information within layer 5 pyramidal neurons during an active sensing behaviour. Layer 5 pyramidal neurons possess elaborate dendritic arborizations that receive functionally distinct inputs, each targeted to spatially separate regions. At the cellular level, coincident input from these segregated pathways initiates regenerative dendritic electrical events that produce bursts of action potential output and circuits featuring this powerful dendritic nonlinearity can implement computations based on input correlation. To examine this in vivo we recorded dendritic activity in layer 5 pyramidal neurons in the barrel cortex using two-photon calcium imaging in mice performing an object-localization task. Large-amplitude, global calcium signals were observed throughout the apical tuft dendrites when active touch occurred at particular object locations or whisker angles. Such global calcium signals are produced by dendritic plateau potentials that require both vibrissal sensory input and primary motor cortex activity. These data provide direct evidence of nonlinear dendritic processing of correlated sensory and motor information in the mammalian neocortex during active sensation.
Neurodata Without Borders: Neurophysiology (NWB:N) is a data standard for neurophysiology, providing neuroscientists with a common standard to share, archive, use, and build common analysis tools for neurophysiology data. With NWB:N version 2.0 (NWB:N 2.0) we made significant advances towards creating a usable standard, software ecosystem, and vibrant community for standardizing neurophysiology data. In this manuscript we focus in particular on the NWB:N data standard schema and present advances towards creating an accessible data standard for neurophysiology.
Genetically encoded calcium indicators (GECIs) are powerful tools for systems neuroscience. Recent efforts in protein engineering have significantly increased the performance of GECIs. The state-of-the art single-wavelength GECI, GCaMP3, has been deployed in a number of model organisms and can reliably detect three or more action potentials in short bursts in several systems in vivo . Through protein structure determination, targeted mutagenesis, high-throughput screening, and a battery of in vitro assays, we have increased the dynamic range of GCaMP3 by severalfold, creating a family of “GCaMP5” sensors. We tested GCaMP5s in several systems: cultured neurons and astrocytes, mouse retina, and in vivo in Caenorhabditis chemosensory neurons, Drosophila larval neuromuscular junction and adult antennal lobe, zebrafish retina and tectum, and mouse visual cortex. Signal-to-noise ratio was improved by at least 2- to 3-fold. In the visual cortex, two GCaMP5 variants detected twice as many visual stimulus-responsive cells as GCaMP3. By combining in vivo imaging with electrophysiology we show that GCaMP5 fluorescence provides a more reliable measure of neuronal activity than its predecessor GCaMP3.GCaMP5allows more sensitive detection of neural activity in vivo andmayfind widespread applications for cellular imaging in general.
Determining how long-range synaptic inputs engage pyramidal neurons in primary motor cortex (M1) is important for understanding circuit mechanisms involved in regulating movement. We used channelrhodopsin-2-assisted circuit mapping to characterize the long-range excitatory synaptic connections made by multiple cortical and thalamic areas onto pyramidal neurons in mouse vibrissal motor cortex (vM1). Each projection innervated vM1 pyramidal neurons with a unique laminar profile. Collectively, the profiles for different sources of input partially overlapped and spanned all cortical layers. Specifically, orbital cortex (OC) inputs primarily targeted neurons in L6. Secondary motor cortex (M2) inputs excited neurons mainly in L5B, including pyramidal tract neurons. In contrast, thalamocortical inputs from anterior motor-related thalamic regions, including VA/VL (ventral anterior thalamic nucleus/ventrolateral thalamic nucleus), targeted neurons in L2/3 through L5B, but avoided L6. Inputs from posterior sensory-related thalamic areas, including POm (posterior thalamic nuclear group), targeted neurons only in the upper layers (L2/3 and L5A), similar to inputs from somatosensory (barrel) cortex. Our results show that long-range excitatory inputs target vM1 pyramidal neurons in a layer-specific manner. Inputs from sensory-related cortical and thalamic areas preferentially target the upper-layer pyramidal neurons in vM1. In contrast, inputs from OC and M2, areas associated with volitional and cognitive aspects of movements, bypass local circuitry and have direct monosynaptic access to neurons projecting to brainstem and thalamus.
Perceptual decision making is an active process where animals move their sense organs to extract task-relevant information. To investigate how the brain translates sensory input into decisions during active sensation, we developed a mouse active touch task where the mechanosensory input can be precisely measured and that challenges animals to use multiple mechanosensory cues. Male mice were trained to localise a pole using a single whisker and to report their decision by selecting one of three choices. Using high-speed imaging and machine vision we estimated whisker-object mechanical forces at millisecond resolution. Mice solved the task by a sensory-motor strategy where both the strength and direction of whisker bending were informative cues to pole location. We found competing influences of immediate sensory input and choice memory on mouse choice. On correct trials, choice could be predicted from the direction and strength of whisker bending, but not from previous choice. In contrast, on error trials, choice could be predicted from previous choice but not from whisker bending. This study shows that animal choices during active tactile decision making can be predicted from mechanosenory and choice-memory signals; and provides a new task, well-suited for future study of the neural basis of active perceptual decisions.Due to the difficulty of measuring the sensory input to moving sense organs, active perceptual decision making remains poorly understood. The whisker system provides a way forward since it is now possible to measure the mechanical forces due to whisker-object contact during behaviour. Here we train mice in a novel behavioural task that challenges them to use rich mechanosensory cues, but can be performed using one whisker and enables task-relevant mechanical forces to be precisely estimated. This approach enables rigorous study of how sensory cues translate into action during active, perceptual decision making. Our findings provide new insight into active touch and how sensory/internal signals interact to determine behavioural choices.