Filter
Associated Lab
- Aguilera Castrejon Lab (2) Apply Aguilera Castrejon Lab filter
- Ahrens Lab (57) Apply Ahrens Lab filter
- Aso Lab (42) Apply Aso Lab filter
- Baker Lab (19) Apply Baker Lab filter
- Betzig Lab (103) Apply Betzig Lab filter
- Beyene Lab (9) Apply Beyene Lab filter
- Bock Lab (14) Apply Bock Lab filter
- Branson Lab (51) Apply Branson Lab filter
- Card Lab (37) Apply Card Lab filter
- Cardona Lab (45) Apply Cardona Lab filter
- Chklovskii Lab (10) Apply Chklovskii Lab filter
- Clapham Lab (14) Apply Clapham Lab filter
- Cui Lab (19) Apply Cui Lab filter
- Darshan Lab (8) Apply Darshan Lab filter
- Dickson Lab (32) Apply Dickson Lab filter
- Druckmann Lab (21) Apply Druckmann Lab filter
- Dudman Lab (40) Apply Dudman Lab filter
- Eddy/Rivas Lab (30) Apply Eddy/Rivas Lab filter
- Egnor Lab (4) Apply Egnor Lab filter
- Espinosa Medina Lab (17) Apply Espinosa Medina Lab filter
- Feliciano Lab (10) Apply Feliciano Lab filter
- Fetter Lab (31) Apply Fetter Lab filter
- FIB-SEM Technology (1) Apply FIB-SEM Technology filter
- Fitzgerald Lab (16) Apply Fitzgerald Lab filter
- Freeman Lab (15) Apply Freeman Lab filter
- Funke Lab (41) Apply Funke Lab filter
- Gonen Lab (59) Apply Gonen Lab filter
- Grigorieff Lab (34) Apply Grigorieff Lab filter
- Harris Lab (54) Apply Harris Lab filter
- Heberlein Lab (13) Apply Heberlein Lab filter
- Hermundstad Lab (25) Apply Hermundstad Lab filter
- Hess Lab (76) Apply Hess Lab filter
- Ilanges Lab (2) Apply Ilanges Lab filter
- Jayaraman Lab (43) Apply Jayaraman Lab filter
- Ji Lab (33) Apply Ji Lab filter
- Johnson Lab (1) Apply Johnson Lab filter
- Karpova Lab (13) Apply Karpova Lab filter
- Keleman Lab (8) Apply Keleman Lab filter
- Keller Lab (61) Apply Keller Lab filter
- Koay Lab (2) Apply Koay Lab filter
- Lavis Lab (142) Apply Lavis Lab filter
- Lee (Albert) Lab (29) Apply Lee (Albert) Lab filter
- Leonardo Lab (19) Apply Leonardo Lab filter
- Li Lab (6) Apply Li Lab filter
- Lippincott-Schwartz Lab (106) Apply Lippincott-Schwartz Lab filter
- Liu (Yin) Lab (2) Apply Liu (Yin) Lab filter
- Liu (Zhe) Lab (59) Apply Liu (Zhe) Lab filter
- Looger Lab (137) Apply Looger Lab filter
- Magee Lab (31) Apply Magee Lab filter
- Menon Lab (12) Apply Menon Lab filter
- Murphy Lab (6) Apply Murphy Lab filter
- O'Shea Lab (6) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Pachitariu Lab (37) Apply Pachitariu Lab filter
- Pastalkova Lab (5) Apply Pastalkova Lab filter
- Pavlopoulos Lab (7) Apply Pavlopoulos Lab filter
- Pedram Lab (4) Apply Pedram Lab filter
- Podgorski Lab (16) Apply Podgorski Lab filter
- Reiser Lab (47) Apply Reiser Lab filter
- Riddiford Lab (20) Apply Riddiford Lab filter
- Romani Lab (36) Apply Romani Lab filter
- Rubin Lab (109) Apply Rubin Lab filter
- Saalfeld Lab (47) Apply Saalfeld Lab filter
- Satou Lab (1) Apply Satou Lab filter
- Scheffer Lab (38) Apply Scheffer Lab filter
- Schreiter Lab (51) Apply Schreiter Lab filter
- Sgro Lab (1) Apply Sgro Lab filter
- Shroff Lab (31) Apply Shroff Lab filter
- Simpson Lab (18) Apply Simpson Lab filter
- Singer Lab (37) Apply Singer Lab filter
- Spruston Lab (60) Apply Spruston Lab filter
- Stern Lab (75) Apply Stern Lab filter
- Sternson Lab (47) Apply Sternson Lab filter
- Stringer Lab (36) Apply Stringer Lab filter
- Svoboda Lab (131) Apply Svoboda Lab filter
- Tebo Lab (11) Apply Tebo Lab filter
- Tervo Lab (9) Apply Tervo Lab filter
- Tillberg Lab (18) Apply Tillberg Lab filter
- Tjian Lab (17) Apply Tjian Lab filter
- Truman Lab (58) Apply Truman Lab filter
- Turaga Lab (41) Apply Turaga Lab filter
- Turner Lab (28) Apply Turner Lab filter
- Vale Lab (8) Apply Vale Lab filter
- Voigts Lab (3) Apply Voigts Lab filter
- Wang (Meng) Lab (25) Apply Wang (Meng) Lab filter
- Wang (Shaohe) Lab (6) Apply Wang (Shaohe) Lab filter
- Wu Lab (8) Apply Wu Lab filter
- Zlatic Lab (26) Apply Zlatic Lab filter
- Zuker Lab (5) Apply Zuker Lab filter
Associated Project Team
- CellMap (12) Apply CellMap filter
- COSEM (3) Apply COSEM filter
- FIB-SEM Technology (5) Apply FIB-SEM Technology filter
- Fly Descending Interneuron (12) Apply Fly Descending Interneuron filter
- Fly Functional Connectome (14) Apply Fly Functional Connectome filter
- Fly Olympiad (5) Apply Fly Olympiad filter
- FlyEM (56) Apply FlyEM filter
- FlyLight (50) Apply FlyLight filter
- GENIE (47) Apply GENIE filter
- Integrative Imaging (6) Apply Integrative Imaging filter
- Larval Olympiad (2) Apply Larval Olympiad filter
- MouseLight (18) Apply MouseLight filter
- NeuroSeq (1) Apply NeuroSeq filter
- ThalamoSeq (1) Apply ThalamoSeq filter
- Tool Translation Team (T3) (28) Apply Tool Translation Team (T3) filter
- Transcription Imaging (45) Apply Transcription Imaging filter
Associated Support Team
- Project Pipeline Support (5) Apply Project Pipeline Support filter
- Anatomy and Histology (18) Apply Anatomy and Histology filter
- Cryo-Electron Microscopy (40) Apply Cryo-Electron Microscopy filter
- Electron Microscopy (18) Apply Electron Microscopy filter
- Gene Targeting and Transgenics (11) Apply Gene Targeting and Transgenics filter
- High Performance Computing (7) Apply High Performance Computing filter
- Integrative Imaging (18) Apply Integrative Imaging filter
- Invertebrate Shared Resource (40) Apply Invertebrate Shared Resource filter
- Janelia Experimental Technology (37) Apply Janelia Experimental Technology filter
- Management Team (1) Apply Management Team filter
- Mass Spectrometry (1) Apply Mass Spectrometry filter
- Molecular Genomics (15) Apply Molecular Genomics filter
- Primary & iPS Cell Culture (14) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (51) Apply Project Technical Resources filter
- Quantitative Genomics (20) Apply Quantitative Genomics filter
- Scientific Computing (96) Apply Scientific Computing filter
- Viral Tools (14) Apply Viral Tools filter
- Vivarium (7) Apply Vivarium filter
Publication Date
- 2025 (210) Apply 2025 filter
- 2024 (211) Apply 2024 filter
- 2023 (157) Apply 2023 filter
- 2022 (166) Apply 2022 filter
- 2021 (175) Apply 2021 filter
- 2020 (177) Apply 2020 filter
- 2019 (177) Apply 2019 filter
- 2018 (206) Apply 2018 filter
- 2017 (186) Apply 2017 filter
- 2016 (191) Apply 2016 filter
- 2015 (195) Apply 2015 filter
- 2014 (190) Apply 2014 filter
- 2013 (136) Apply 2013 filter
- 2012 (112) Apply 2012 filter
- 2011 (98) Apply 2011 filter
- 2010 (61) Apply 2010 filter
- 2009 (56) Apply 2009 filter
- 2008 (40) Apply 2008 filter
- 2007 (21) Apply 2007 filter
- 2006 (3) Apply 2006 filter
2768 Janelia Publications
Showing 1731-1740 of 2768 resultsBACKGROUND: The use of genetically-encoded fluorescent reporters is essential for the identification and observation of cells that express transgenic modulatory proteins. Near-infrared (NIR) fluorescent proteins have superior light penetration through biological tissue, but are not yet widely adopted. NEW METHOD: Using the near-infrared fluorescent protein, iRFP713, improves the imaging resolution in thick tissue sections or the intact brain due to the reduced light-scattering at the longer, NIR wavelengths used to image the protein. Additionally, iRFP713 can be used to identify transgenic cells without photobleaching other fluorescent reporters or affecting opsin function. We have generated a set of adeno-associated vectors in which iRFP713 has been fused to optogenetic channels, and can be expressed constitutively or Cre-dependently. RESULTS: iRFP713 is detectable when expressed in neurons both in vitro and in vivo without exogenously supplied chromophore biliverdin. Neuronally-expressed iRFP713 has similar properties to GFP-like fluorescent proteins, including the ability to be translationally fused to channelrhodopsin or halorhodopsin, however, it shows superior photostability compared to EYFP. Furthermore, electrophysiological recordings from iRFP713-labeled cells compared to cells labeled with mCherry suggest that iRFP713 cells are healthier and therefore more stable and reliable in an ex vivo preparation. Lastly, we have generated a transgenic rat that expresses iRFP713 in a Cre-dependent manner. CONCLUSIONS: Overall, we have demonstrated that iRFP713 can be used as a reporter in neurons without the use of exogenous biliverdin, with minimal impact on viability and function thereby making it feasible to extend the capabilities for imaging genetically-tagged neurons in slices and in vivo.
Oxytocin plays a critical role in regulating social behaviors, yet our understanding of its function in both neurological health and disease remains incomplete. Real-time oxytocin imaging probes with spatiotemporal resolution relevant to its endogenous signaling are required to fully elucidate oxytocin's role in the brain. Herein, we describe a near-infrared oxytocin nanosensor (nIROXT), a synthetic probe capable of imaging oxytocin in the brain without interference from its structural analogue, vasopressin. nIROXT leverages the inherent tissue-transparent fluorescence of single-walled carbon nanotubes (SWCNT) and the molecular recognition capacity of an oxytocin receptor peptide fragment to selectively and reversibly image oxytocin. We employ these nanosensors to monitor electrically stimulated oxytocin release in brain tissue, revealing oxytocin release sites with a median size of 3 µm in the paraventricular nucleus of C57BL/6 mice, which putatively represents the spatial diffusion of oxytocin from its point of release. These data demonstrate that covalent SWCNT constructs, such as nIROXT, are powerful optical tools that can be leveraged to measure neuropeptide release in brain tissue.
Imaging approaches based on single molecule localization break the diffraction barrier of conventional fluorescence microscopy, allowing for bioimaging with nanometer resolution. It remains a challenge, however, to precisely localize photon-limited single molecules in 3D. We have developed a new localization-based imaging technique achieving almost isotropic subdiffraction resolution in 3D. A tilted mirror is used to generate a side view in addition to the front view of activated single emitters, allowing their 3D localization to be precisely determined for superresolution imaging. Because both front and side views are in focus, this method is able to efficiently collect emitted photons. The technique is simple to implement on a commercial fluorescence microscope, and especially suitable for biological samples with photon-limited chromophores such as endogenously expressed photoactivatable fluorescent proteins. Moreover, this method is relatively resistant to optical aberration, as it requires only centroid determination for localization analysis. Here we demonstrate the application of this method to 3D imaging of bacterial protein distribution and neuron dendritic morphology with subdiffraction resolution.
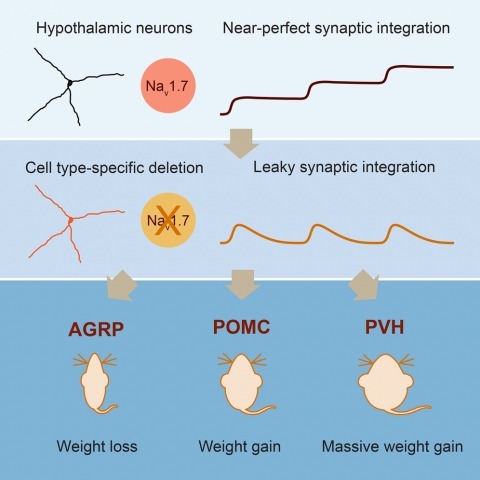
Neurons are well suited for computations on millisecond timescales, but some neuronal circuits set behavioral states over long time periods, such as those involved in energy homeostasis. We found that multiple types of hypothalamic neurons, including those that oppositely regulate body weight, are specialized as near-perfect synaptic integrators that summate inputs over extended timescales. Excitatory postsynaptic potentials (EPSPs) are greatly prolonged, outlasting the neuronal membrane time-constant up to 10-fold. This is due to the voltage-gated sodium channel Nav1.7 (Scn9a), previously associated with pain-sensation but not synaptic integration. Scn9a deletion in AGRP, POMC, or paraventricular hypothalamic neurons reduced EPSP duration, synaptic integration, and altered body weight in mice. In vivo whole-cell recordings in the hypothalamus confirmed near-perfect synaptic integration. These experiments show that integration of synaptic inputs over time by Nav1.7 is critical for body weight regulation and reveal a mechanism for synaptic control of circuits regulating long term homeostatic functions.
Perceptual success depends on fast-spiking, parvalbumin-positive interneurons (FS/PVs). However, competing theories of optimal rate and correlation in pyramidal (PYR) firing make opposing predictions regarding the underlying FS/PV dynamics. We addressed this with population calcium imaging of FS/PVs and putative PYR neurons during threshold detection. In primary somatosensory and visual neocortex, a distinct PYR subset shows increased rate and spike-count correlations on detected trials ("hits"), while most show no rate change and decreased correlations. A larger fraction of FS/PVs predicts hits with either rate increases or decreases. Using computational modeling, we found that inhibitory imbalance, created by excitatory "feedback" and interactions between FS/PV pools, can account for the data. Rate-decreasing FS/PVs increase rate and correlation in a PYR subset, while rate-increasing FS/PVs reduce correlations and offset enhanced excitation in PYR neurons. These findings indicate that selection of informative PYR ensembles, through transient inhibitory imbalance, is a common motif of optimal neocortical processing.
Many motor control systems generate multiple movements using a common set of muscles. How are premotor circuits able to flexibly generate diverse movement patterns? Here, we characterize the neuronal circuits that drive the distinct courtship songs of Drosophila melanogaster. Male flies vibrate their wings towards females to produce two different song modes – pulse and sine song – which signal species identity and male quality. Using cell-type specific genetic reagents and the connectome, we provide a cellular and synaptic map of the circuits in the male ventral nerve cord that generate these songs and examine how activating or inhibiting each cell type within these circuits affects the song. Our data reveal that the song circuit is organized into two nested feed-forward pathways, with extensive reciprocal and feed-back connections. The larger network produces pulse song, the more complex and ancestral song form. A subset of this network produces sine song, the simpler and more recent form. Such nested organization may be a common feature of motor control circuits in which evolution has layered increasing flexibility on to a basic movement pattern.
Although hippocampal theta oscillations represent a prime example of temporal coding in the mammalian brain, little is known about the specific biophysical mechanisms. Intracellular recordings support a particular abstract oscillatory interference model of hippocampal theta activity, the soma-dendrite interference model. To gain insight into the cellular and circuit level mechanisms of theta activity, we implemented a similar form of interference using the actual hippocampal network in mice in vitro. We found that pairing increasing levels of phasic dendritic excitation with phasic stimulation of perisomatic projecting inhibitory interneurons induced a somatic polarization and action potential timing profile that reproduced most common features. Alterations in the temporal profile of inhibition were required to fully capture all features. These data suggest that theta-related place cell activity is generated through an interaction between a phasic dendritic excitation and a phasic perisomatic shunting inhibition delivered by interneurons, a subset of which undergo activity-dependent presynaptic modulation.
Regions within the prefrontal cortex are thought to process beliefs about the world, but little is known about the circuit dynamics underlying the formation and modification of these beliefs. Using a task that permits dissociation between the activity encoding an animal’s internal state and that encoding aspects of behavior, we found that transient increases in the volatility of activity in the rat medial prefrontal cortex accompany periods when an animal’s belief is modified after an environmental change. Activity across the majority of sampled neurons underwent marked, abrupt, and coordinated changes when prior belief was abandoned in favor of exploration of alternative strategies. These dynamics reflect network switches to a state of instability, which diminishes over the period of exploration as new stable representations are formed.
Brains comprise complex networks of neurons and connections, similar to the nodes and edges of artificial networks. Network analysis applied to the wiring diagrams of brains can offer insights into how they support computations and regulate the flow of information underlying perception and behaviour. The completion of the first whole-brain connectome of an adult fly, containing over 130,000 neurons and millions of synaptic connections, offers an opportunity to analyse the statistical properties and topological features of a complete brain. Here we computed the prevalence of two- and three-node motifs, examined their strengths, related this information to both neurotransmitter composition and cell type annotations, and compared these metrics with wiring diagrams of other animals. We found that the network of the fly brain displays rich-club organization, with a large population (30% of the connectome) of highly connected neurons. We identified subsets of rich-club neurons that may serve as integrators or broadcasters of signals. Finally, we examined subnetworks based on 78 anatomically defined brain regions or neuropils. These data products are shared within the FlyWire Codex (https://codex.flywire.ai) and should serve as a foundation for models and experiments exploring the relationship between neural activity and anatomical structure.
We give a covering number bound for deep learning networks that is independent of the size of the network. The key for the simple analysis is that for linear classifiers, rotating the data doesn't affect the covering number. Thus, we can ignore the rotation part of each layer's linear transformation, and get the covering number bound by concentrating on the scaling part.