Filter
Associated Lab
- Ahrens Lab (5) Apply Ahrens Lab filter
- Aso Lab (3) Apply Aso Lab filter
- Betzig Lab (7) Apply Betzig Lab filter
- Bock Lab (5) Apply Bock Lab filter
- Branson Lab (3) Apply Branson Lab filter
- Card Lab (2) Apply Card Lab filter
- Cardona Lab (4) Apply Cardona Lab filter
- Clapham Lab (2) Apply Clapham Lab filter
- Darshan Lab (1) Apply Darshan Lab filter
- Dickson Lab (5) Apply Dickson Lab filter
- Druckmann Lab (3) Apply Druckmann Lab filter
- Dudman Lab (4) Apply Dudman Lab filter
- Espinosa Medina Lab (3) Apply Espinosa Medina Lab filter
- Feliciano Lab (1) Apply Feliciano Lab filter
- Fitzgerald Lab (2) Apply Fitzgerald Lab filter
- Funke Lab (1) Apply Funke Lab filter
- Gonen Lab (2) Apply Gonen Lab filter
- Grigorieff Lab (4) Apply Grigorieff Lab filter
- Harris Lab (4) Apply Harris Lab filter
- Heberlein Lab (2) Apply Heberlein Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Hess Lab (5) Apply Hess Lab filter
- Jayaraman Lab (4) Apply Jayaraman Lab filter
- Ji Lab (1) Apply Ji Lab filter
- Johnson Lab (1) Apply Johnson Lab filter
- Keleman Lab (2) Apply Keleman Lab filter
- Keller Lab (6) Apply Keller Lab filter
- Lavis Lab (6) Apply Lavis Lab filter
- Lee (Albert) Lab (1) Apply Lee (Albert) Lab filter
- Lippincott-Schwartz Lab (12) Apply Lippincott-Schwartz Lab filter
- Liu (Zhe) Lab (7) Apply Liu (Zhe) Lab filter
- Looger Lab (15) Apply Looger Lab filter
- O'Shea Lab (1) Apply O'Shea Lab filter
- Pachitariu Lab (4) Apply Pachitariu Lab filter
- Pavlopoulos Lab (1) Apply Pavlopoulos Lab filter
- Podgorski Lab (4) Apply Podgorski Lab filter
- Reiser Lab (2) Apply Reiser Lab filter
- Romani Lab (3) Apply Romani Lab filter
- Rubin Lab (6) Apply Rubin Lab filter
- Saalfeld Lab (3) Apply Saalfeld Lab filter
- Scheffer Lab (2) Apply Scheffer Lab filter
- Schreiter Lab (4) Apply Schreiter Lab filter
- Simpson Lab (1) Apply Simpson Lab filter
- Singer Lab (4) Apply Singer Lab filter
- Spruston Lab (6) Apply Spruston Lab filter
- Stern Lab (5) Apply Stern Lab filter
- Sternson Lab (2) Apply Sternson Lab filter
- Stringer Lab (3) Apply Stringer Lab filter
- Svoboda Lab (14) Apply Svoboda Lab filter
- Tillberg Lab (2) Apply Tillberg Lab filter
- Truman Lab (4) Apply Truman Lab filter
- Turaga Lab (2) Apply Turaga Lab filter
- Turner Lab (2) Apply Turner Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Associated Support Team
- Cryo-Electron Microscopy (4) Apply Cryo-Electron Microscopy filter
- Electron Microscopy (4) Apply Electron Microscopy filter
- Gene Targeting and Transgenics (2) Apply Gene Targeting and Transgenics filter
- Integrative Imaging (1) Apply Integrative Imaging filter
- Invertebrate Shared Resource (5) Apply Invertebrate Shared Resource filter
- Janelia Experimental Technology (6) Apply Janelia Experimental Technology filter
- Molecular Genomics (1) Apply Molecular Genomics filter
- Primary & iPS Cell Culture (1) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (4) Apply Project Technical Resources filter
- Quantitative Genomics (4) Apply Quantitative Genomics filter
- Scientific Computing Software (5) Apply Scientific Computing Software filter
- Scientific Computing Systems (1) Apply Scientific Computing Systems filter
Publication Date
- December 2019 (9) Apply December 2019 filter
- November 2019 (11) Apply November 2019 filter
- October 2019 (18) Apply October 2019 filter
- September 2019 (15) Apply September 2019 filter
- August 2019 (14) Apply August 2019 filter
- July 2019 (12) Apply July 2019 filter
- June 2019 (18) Apply June 2019 filter
- May 2019 (12) Apply May 2019 filter
- April 2019 (16) Apply April 2019 filter
- March 2019 (17) Apply March 2019 filter
- February 2019 (18) Apply February 2019 filter
- January 2019 (17) Apply January 2019 filter
- Remove 2019 filter 2019
177 Janelia Publications
Showing 11-20 of 177 resultsWe compared performance of recently developed silicon photomultipliers (SiPMs) to GaAsP photomultiplier tubes (PMTs) for two-photon imaging of neural activity. Despite higher dark counts, SiPMs match or exceed the signal-to-noise ratio of PMTs at photon rates encountered in typical calcium imaging experiments due to their low pulse height variability. At higher photon rates encountered during high-speed voltage imaging, SiPMs substantially outperform PMTs.
External cues, including touch, enable walking animals to flexibly maneuver around obstacles and extricate themselves from dead-ends (for reviews, see [1-3]). In a screen for neurons that enable Drosophila melanogaster to retreat when it encounters a dead-end, we identified a pair of ascending neurons, the TwoLumps Ascending (TLA) neurons. Silencing TLA activity impairs backward locomotion, whereas optogenetic activation triggers backward walking. TLA-induced reversal is mediated in part by the Moonwalker Descending Neurons (MDNs) [4], which receive excitatory input from the TLAs. Silencing the TLAs decreases the extent to which freely walking flies back up upon encountering a physical barrier in the dark, and TLAs show calcium responses to optogenetic activation of neurons expressing the mechanosensory channel NOMPC. We infer that TLAs convey feedforward mechanosensory stimuli to transiently activate MDNs in response to anterior body touch.
Many animals rely on an internal heading representation when navigating in varied environments. How this representation is linked to the sensory cues that define different surroundings is unclear. In the fly brain, heading is represented by 'compass' neurons that innervate a ring-shaped structure known as the ellipsoid body. Each compass neuron receives inputs from 'ring' neurons that are selective for particular visual features; this combination provides an ideal substrate for the extraction of directional information from a visual scene. Here we combine two-photon calcium imaging and optogenetics in tethered flying flies with circuit modelling, and show how the correlated activity of compass and visual neurons drives plasticity, which flexibly transforms two-dimensional visual cues into a stable heading representation. We also describe how this plasticity enables the fly to convert a partial heading representation, established from orienting within part of a novel setting, into a complete heading representation. Our results provide mechanistic insight into the memory-related computations that are essential for flexible navigation in varied surroundings.
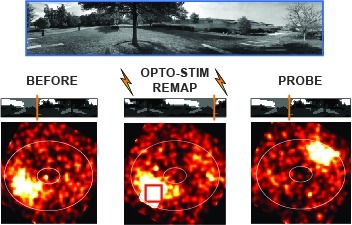
Optogenetics allows manipulations of genetically and spatially defined neuronal populations with excellent temporal control. However, neurons are coupled with other neurons over multiple length scales, and the effects of localized manipulations thus spread beyond the targeted neurons. We benchmarked several optogenetic methods to inactivate small regions of neocortex. Optogenetic excitation of GABAergic neurons produced more effective inactivation than light-gated ion pumps. Transgenic mice expressing the light-dependent chloride channel GtACR1 produced the most potent inactivation. Generally, inactivation spread substantially beyond the photostimulation light, caused by strong coupling between cortical neurons. Over some range of light intensity, optogenetic excitation of inhibitory neurons reduced activity in these neurons, together with pyramidal neurons, a signature of inhibition-stabilized neural networks ('paradoxical effect'). The offset of optogenetic inactivation was followed by rebound excitation in a light dose-dependent manner, limiting temporal resolution. Our data offer guidance for the design of optogenetics experiments.
Animals employ diverse learning rules and synaptic plasticity dynamics to record temporal and statistical information about the world. However, the molecular mechanisms underlying this diversity are poorly understood. The anatomically defined compartments of the insect mushroom body function as parallel units of associative learning, with different learning rates, memory decay dynamics and flexibility (Aso & Rubin 2016). Here we show that nitric oxide (NO) acts as a neurotransmitter in a subset of dopaminergic neurons in . NO's effects develop more slowly than those of dopamine and depend on soluble guanylate cyclase in postsynaptic Kenyon cells. NO acts antagonistically to dopamine; it shortens memory retention and facilitates the rapid updating of memories. The interplay of NO and dopamine enables memories stored in local domains along Kenyon cell axons to be specialized for predicting the value of odors based only on recent events. Our results provide key mechanistic insights into how diverse memory dynamics are established in parallel memory systems.
The target for the "rapid" (<24 h) antidepressant effects of S-ketamine is unknown, vitiating programs to rationally develop more effective rapid antidepressants. To describe a drug's target, one must first understand the compartments entered by the drug, at all levels-the organ, the cell, and the organelle. We have, therefore, developed molecular tools to measure the subcellular, organellar pharmacokinetics of S-ketamine. The tools are genetically encoded intensity-based S-ketamine-sensing fluorescent reporters, iSKetSnFR1 and iSKetSnFR2. In solution, these biosensors respond to S-ketamine with a sensitivity, S-slope = delta(F/F)/(delta[S-ketamine]) of 0.23 and 1.9/μM, respectively. The iSKetSnFR2 construct allows measurements at <0.3 μM S-ketamine. The iSKetSnFR1 and iSKetSnFR2 biosensors display >100-fold selectivity over other ligands tested, including R-ketamine. We targeted each of the sensors to either the plasma membrane (PM) or the endoplasmic reticulum (ER). Measurements on these biosensors expressed in Neuro2a cells and in human dopaminergic neurons differentiated from induced pluripotent stem cells (iPSCs) show that S-ketamine enters the ER within a few seconds after appearing in the external solution near the PM, then leaves as rapidly after S-ketamine is removed from the extracellular solution. In cells, S-slopes for the ER and PM-targeted sensors differ by <2-fold, indicating that the ER [S-ketamine] is less than 2-fold different from the extracellular [S-ketamine]. Organelles represent potential compartments for the engagement of S-ketamine with its antidepressant target, and potential S-ketamine targets include organellar ion channels, receptors, and transporters.
In pursuit of food, hungry animals mobilize significant energy resources and overcome exhaustion and fear. How need and motivation control the decision to continue or change behavior is not understood. Using a single fly treadmill, we show that hungry flies persistently track a food odor and increase their effort over repeated trials in the absence of reward suggesting that need dominates negative experience. We further show that odor tracking is regulated by two mushroom body output neurons (MBONs) connecting the MB to the lateral horn. These MBONs, together with dopaminergic neurons and Dop1R2 signaling, control behavioral persistence. Conversely, an octopaminergic neuron, VPM4, which directly innervates one of the MBONs, acts as a brake on odor tracking by connecting feeding and olfaction. Together, our data suggest a function for the MB in internal state-dependent expression of behavior that can be suppressed by external inputs conveying a competing behavioral drive.
R7 UV photoreceptors (PRs) are divided into yellow (y) and pale (p) subtypes. yR7 PRs express the Dpr11 cell surface protein and are presynaptic to Dm8 amacrine neurons (yDm8) that express Dpr11's binding partner DIP-g, while pR7 PRs synapse onto DIP-g-negative pDm8. Dpr11 and DIP-g expression patterns define 'yellow' and 'pale' color vision circuits. We examined Dm8 neurons in these circuits by electron microscopic reconstruction and expansion microscopy. and mutations affect the morphologies of yDm8 distal ('home column') dendrites. yDm8 neurons are generated in excess during development and compete for presynaptic yR7 PRs, and interactions between Dpr11 and DIP-g are required for yDm8 survival. These interactions also allow yDm8 neurons to select yR7 PRs as their appropriate home column partners. yDm8 and pDm8 neurons do not normally compete for survival signals or R7 partners, but can be forced to do so by manipulation of R7 subtype fate.
Brains are notoriously hard to understand, and neuroscientists need all the tools they can get their hands on to have a realistic shot at it. Advances in machine learning are proving instrumental, illustrated by their recent use to shed light on navigational strategies implemented by zebrafish brains.
3-photon excitation enables fluorescence microscopy deep in densely labeled and highly scattering samples. To date, 3-photon excitation has been restricted to scanning a single focus, limiting the speed of volume acquisition. Here, for the first time to our knowledge, we implemented and characterized dual-plane 3-photon microscopy with temporal multiplexing and remote focusing, and performed simultaneous calcium imaging of two planes beyond 600 µm deep in the cortex of a pan-excitatory GCaMP6s transgenic mouse with a per-plane framerate of 7 Hz and an effective 2 MHz laser repetition rate. This method is a straightforward and generalizable modification to single-focus 3PE systems, doubling the rate of volume (column) imaging with off-the-shelf components and minimal technical constraints.