Filter
Associated Lab
- Dudman Lab (1) Apply Dudman Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Lavis Lab (1) Apply Lavis Lab filter
- Lee (Albert) Lab (1) Apply Lee (Albert) Lab filter
- Looger Lab (2) Apply Looger Lab filter
- Pachitariu Lab (1) Apply Pachitariu Lab filter
- Saalfeld Lab (2) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Remove Sternson Lab filter Sternson Lab
- Svoboda Lab (4) Apply Svoboda Lab filter
- Tillberg Lab (3) Apply Tillberg Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
Associated Project Team
Publication Date
- 2023 (2) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (3) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (2) Apply 2019 filter
- 2017 (2) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (7) Apply 2015 filter
- 2014 (5) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (4) Apply 2012 filter
- 2011 (6) Apply 2011 filter
- 2010 (1) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2008 (1) Apply 2008 filter
- 2005 (1) Apply 2005 filter
- 2004 (1) Apply 2004 filter
- 2002 (1) Apply 2002 filter
- 2001 (2) Apply 2001 filter
- 1998 (1) Apply 1998 filter
Type of Publication
54 Publications
Showing 41-50 of 54 resultsHomeostasis is a biological principle for regulation of essential physiological parameters within a set range. Behavioural responses due to deviation from homeostasis are critical for survival, but motivational processes engaged by physiological need states are incompletely understood. We examined motivational characteristics of two separate neuron populations that regulate energy and fluid homeostasis by using cell-type-specific activity manipulations in mice. We found that starvation-sensitive AGRP neurons exhibit properties consistent with a negative-valence teaching signal. Mice avoided activation of AGRP neurons, indicating that AGRP neuron activity has negative valence. AGRP neuron inhibition conditioned preference for flavours and places. Correspondingly, deep-brain calcium imaging revealed that AGRP neuron activity rapidly reduced in response to food-related cues. Complementary experiments activating thirst-promoting neurons also conditioned avoidance. Therefore, these need-sensing neurons condition preference for environmental cues associated with nutrient or water ingestion, which is learned through reduction of negative-valence signals during restoration of homeostasis.
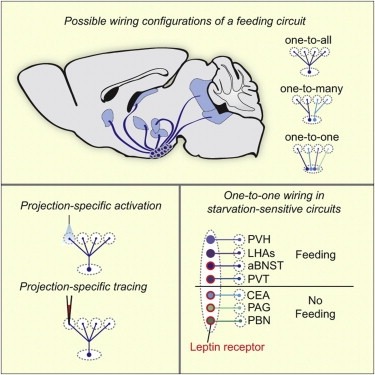
Neural circuits for essential natural behaviors are shaped by selective pressure to coordinate reliable execution of flexible goal-directed actions. However, the structural and functional organization of survival-oriented circuits is poorly understood due to exceptionally complex neuroanatomy. This is exemplified by AGRP neurons, which are a molecularly defined population that is sufficient to rapidly coordinate voracious food seeking and consumption behaviors. Here, we use cell-type-specific techniques for neural circuit manipulation and projection-specific anatomical analysis to examine the organization of this critical homeostatic circuit that regulates feeding. We show that AGRP neuronal circuits use a segregated, parallel, and redundant output configuration. AGRP neuron axon projections that target different brain regions originate from distinct subpopulations, several of which are sufficient to independently evoke feeding. The concerted anatomical and functional analysis of AGRP neuron projection populations reveals a constellation of core forebrain nodes, which are part of an extended circuit that mediates feeding behavior.
The dorsal raphe nucleus (DRN) is an important brain area for body-weight regulation. In this issue of Cell, Nectow et al. uncover cell-type-specific neural circuitry and pharmacology for appetite control within the DRN.
Neuronal cell types are the nodes of neural circuits that determine the flow of information within the brain. Neuronal morphology, especially the shape of the axonal arbor, provides an essential descriptor of cell type and reveals how individual neurons route their output across the brain. Despite the importance of morphology, few projection neurons in the mouse brain have been reconstructed in their entirety. Here we present a robust and efficient platform for imaging and reconstructing complete neuronal morphologies, including axonal arbors that span substantial portions of the brain. We used this platform to reconstruct more than 1,000 projection neurons in the motor cortex, thalamus, subiculum, and hypothalamus. Together, the reconstructed neurons constitute more than 85 meters of axonal length and are available in a searchable online database. Axonal shapes revealed previously unknown subtypes of projection neurons and suggest organizational principles of long-range connectivity.
Transforming synaptic input into action potential output is a fundamental function of neurons. The pattern of action potential output from principal cells of the mammalian hippocampus encodes spatial and nonspatial information, but the cellular and circuit mechanisms by which neurons transform their synaptic input into a given output are unknown. Using a combination of optical activation and cell type-specific pharmacogenetic silencing in vitro, we found that dendritic inhibition is the primary regulator of input-output transformations in mouse hippocampal CA1 pyramidal cells, and acts by gating the dendritic electrogenesis driving burst spiking. Dendrite-targeting interneurons are themselves modulated by interneurons targeting pyramidal cell somata, providing a synaptic substrate for tuning pyramidal cell output through interactions in the local inhibitory network. These results provide evidence for a division of labor in cortical circuits, where distinct computational functions are implemented by subtypes of local inhibitory neurons.
Small molecules are important tools to measure and modulate intracellular signaling pathways. A longstanding limitation for using chemical compounds in complex tissues has been the inability to target bioactive small molecules to a specific cell class. Here, we describe a generalizable esterase-ester pair capable of targeted delivery of small molecules to living cells and tissue with cellular specificity. We used fluorogenic molecules to rapidly identify a small ester masking motif that is stable to endogenous esterases, but is efficiently removed by an exogenous esterase. This strategy allows facile targeting of dyes and drugs in complex biological environments to label specific cell types, illuminate gap junction connectivity, and pharmacologically perturb distinct subsets of cells. We expect this approach to have general utility for the specific delivery of many small molecules to defined cellular populations.
Diversity-oriented organic synthesis offers the promise of advancing chemical genetics, where small molecules are used to explore biology. While the split--pool synthetic method is theoretically the most effective approach for the production of large collections of small molecules, it has not been widely adopted due to numerous technical and analytical hurdles. We have developed a split--pool synthesis leading to an array of stock solutions of single 1,3-dioxanes. The quantities of compounds are sufficient for hundreds of phenotypic and protein-binding assays. The average concentration of these stock solutions derived from a single synthesis bead was determined to be 5.4 mM in 5 microL of DMSO. A mass spectrometric strategy to identify the structure of molecules from a split--pool synthesis was shown to be highly accurate. Individual members of the 1,3-dioxane library have activity in a variety of phenotypic and protein-binding assays. The procedure developed in this study allows many assays to be performed with compounds derived from individual synthesis beads. The synthetic compounds identified in these assays should serve as useful probes of cellular and organismal processes.
Seventy-two hundred potential inhibitors of the histone deacetylase (HDAC) enzyme family, based on a 1,3-dioxane diversity structure, were synthesized on polystyrene macrobeads. The compounds were arrayed for biological assays in a "one bead-one stock solution" format. Metal-chelating functional groups were used to direct the 1,3-dioxanes to HDAC enzymes, which are zinc hydrolases. Representative structures from this library were tested for inhibitory activity and the 1,3-dioxane structure was shown to be compatible with HDAC inhibition. [structure: see text]