Filter
Associated Lab
- Betzig Lab (1) Apply Betzig Lab filter
- Dickson Lab (2) Apply Dickson Lab filter
- Druckmann Lab (1) Apply Druckmann Lab filter
- Funke Lab (1) Apply Funke Lab filter
- Gonen Lab (2) Apply Gonen Lab filter
- Grigorieff Lab (2) Apply Grigorieff Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Jayaraman Lab (1) Apply Jayaraman Lab filter
- Ji Lab (1) Apply Ji Lab filter
- Kainmueller Lab (1) Apply Kainmueller Lab filter
- Lee (Albert) Lab (1) Apply Lee (Albert) Lab filter
- Li Lab (1) Apply Li Lab filter
- Looger Lab (2) Apply Looger Lab filter
- Magee Lab (1) Apply Magee Lab filter
- Podgorski Lab (1) Apply Podgorski Lab filter
- Saalfeld Lab (1) Apply Saalfeld Lab filter
- Stern Lab (1) Apply Stern Lab filter
- Sternson Lab (1) Apply Sternson Lab filter
- Svoboda Lab (1) Apply Svoboda Lab filter
- Truman Lab (1) Apply Truman Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
Associated Project Team
Publication Date
- June 28, 2016 (1) Apply June 28, 2016 filter
- June 27, 2016 (2) Apply June 27, 2016 filter
- June 23, 2016 (1) Apply June 23, 2016 filter
- June 20, 2016 (1) Apply June 20, 2016 filter
- June 17, 2016 (2) Apply June 17, 2016 filter
- June 16, 2016 (3) Apply June 16, 2016 filter
- June 15, 2016 (3) Apply June 15, 2016 filter
- June 14, 2016 (2) Apply June 14, 2016 filter
- June 10, 2016 (1) Apply June 10, 2016 filter
- June 8, 2016 (1) Apply June 8, 2016 filter
- June 7, 2016 (1) Apply June 7, 2016 filter
- June 6, 2016 (1) Apply June 6, 2016 filter
- June 4, 2016 (1) Apply June 4, 2016 filter
- June 3, 2016 (1) Apply June 3, 2016 filter
- June 2, 2016 (1) Apply June 2, 2016 filter
- June 1, 2016 (3) Apply June 1, 2016 filter
- Remove June 2016 filter June 2016
- Remove 2016 filter 2016
Type of Publication
25 Publications
Showing 1-10 of 25 resultsDrosophila larval locomotion, which entails rhythmic body contractions, is controlled by sensory feedback from proprioceptors. The molecular mechanisms mediating this feedback are little understood. By using genetic knock-in and immunostaining, we found that the Drosophila melanogaster transmembrane channel-like (tmc) gene is expressed in the larval class I and class II dendritic arborization (da) neurons and bipolar dendrite (bd) neurons, both of which are known to provide sensory feedback for larval locomotion. Larvae with knockdown or loss of tmc function displayed reduced crawling speeds, increased head cast frequencies, and enhanced backward locomotion. Expressing Drosophila TMC or mammalian TMC1 and/or TMC2 in the tmc-positive neurons rescued these mutant phenotypes. Bending of the larval body activated the tmc-positive neurons, and in tmc mutants this bending response was impaired. This implicates TMC's roles in Drosophila proprioception and the sensory control of larval locomotion. It also provides evidence for a functional conservation between Drosophila and mammalian TMCs.
Segmenting an image into multiple components is a central task in computer vision. In many practical scenarios, prior knowledge about plausible components is available. Incorporating such prior knowledge into models and algorithms for image segmentation is highly desirable, yet can be non-trivial. In this work, we introduce a new approach that allows, for the first time, to constrain some or all components of a segmentation to have convex shapes. Specifically, we extend the Minimum Cost Multicut Problem by a class of constraints that enforce convexity. To solve instances of this NP-hard integer linear program to optimality, we separate the proposed constraints in the branch-and-cut loop of a state-of-the-art ILP solver. Results on photographs and micrographs demonstrate the effectiveness of the approach as well as its advantages over the state-of-the-art heuristic.
Destabilized nanobodies can be used to deliver fluorescent proteins and enzymes to specific targets inside cells.
A 60-year-old man diagnosed with macular telangiectasia type 1 (MacTel 1) was treated for 3 years with monthly aflibercept (Eylea; Regeneron, Tarrytown, NY) and serially imaged with spectral-domain optical coherence tomography. When administered monthly, aflibercept appeared to have a beneficial effect on macular edema secondary to MacTel 1. Visual acuity preservation despite minimal chronic macular edema could be attributed to the lack of significant photoreceptor disruption.
It is unclear how regulatory genes establish neural circuits that compose sex-specific behaviors. The Drosophila melanogaster male courtship song provides a powerful model to study this problem. Courting males vibrate a wing to sing bouts of pulses and hums, called pulse and sine song, respectively. We report the discovery of male-specific thoracic interneurons—the TN1A neurons—that are required specifically for sine song. The TN1A neurons can drive the activity of a sex-non-specific wing motoneuron, hg1, which is also required for sine song. The male-specific connection between the TN1A neurons and the hg1 motoneuron is regulated by the sexual differentiation gene doublesex. We find that doublesex is required in the TN1A neurons during development to increase the density of the TN1A arbors that interact with dendrites of the hg1motoneuron. Our findings demonstrate how a sexual differentiation gene can build a sex-specific circuit motif by modulating neuronal arborization. •Doublesex-expressing TN1 neurons are necessary and sufficient for the male sine song•A subclass of TN1 neurons, TN1A, contributes to the sine song•TN1A neurons are functionally coupled to a sine song motoneuron, hg1•Doublesex regulates the connectivity between the TN1A and hg1 neurons It is unclear how developmental regulatory genes specify sex-specific behaviors. Shirangi et al. demonstrate that the Drosophila sexual differentiation gene doublesex encodes a sex-specific behavior—male song—by promoting the connectivity between the male-specific TN1A neurons and the sex-non-specific hg1 neurons, which are required for production of the song.
Electrical coupling in circuits can produce non-intuitive circuit dynamics, as seen in both experimental work from the crustacean stomatogastric ganglion and in computational models inspired by the connectivity in this preparation. Ambiguities in interpreting the results of electrophysiological recordings can arise if sets of pre- or postsynaptic neurons are electrically coupled, or if the electrical coupling exhibits some specificity (e.g. rectifying, or voltage-dependent). Even in small circuits, electrical coupling can produce parallel pathways that can allow information to travel by monosynaptic and/or polysynaptic pathways. Consequently, similar changes in circuit dynamics can arise from entirely different underlying mechanisms. When neurons are coupled both chemically and electrically, modifying the relative strengths of the two interactions provides a mechanism for flexibility in circuit outputs. This, together with neuromodulation of gap junctions and coupled neurons is important both in developing and adult circuits. This article is protected by copyright. All rights reserved.
Electrical coupling in circuits can produce non-intuitive circuit dynamics, as seen in both experimental work from the crustacean stomatogastric ganglion and in computational models inspired by the connectivity in this preparation. Ambiguities in interpreting the results of electrophysiological recordings can arise if sets of pre- or postsynaptic neurons are electrically coupled, or if the electrical coupling exhibits some specificity (e.g. rectifying, or voltage-dependent). Even in small circuits, electrical coupling can produce parallel pathways that can allow information to travel by monosynaptic and/or polysynaptic pathways. Consequently, similar changes in circuit dynamics can arise from entirely different underlying mechanisms. When neurons are coupled both chemically and electrically, modifying the relative strengths of the two interactions provides a mechanism for flexibility in circuit outputs. This, together with neuromodulation of gap junctions and coupled neurons is important both in developing and adult circuits. This article is protected by copyright. All rights reserved.
Microelectron diffraction (MicroED) is a new cryo-electron microscopy (cryo-EM) method capable of determining macromolecular structures at atomic resolution from vanishingly small 3D crystals. MicroED promises to solve atomic resolution structures from even the tiniest of crystals, less than a few hundred nanometers thick. MicroED complements frontier advances in crystallography and represents part of the rebirth of cryo-EM that is making macromolecular structure determination more accessible for all. Here we review the concept and practice of MicroED, for both the electron microscopist and crystallographer. Where other reviews have addressed specific details of the technique (Hattne et al., 2015, Shi et al., 2016 and Shi et al., 2013), we aim to provide context and highlight important features that should be considered when performing a MicroED experiment.
Following considerable progress on the molecular and cellular basis of taste perception in fly sensory neurons, the time is now ripe to explore how taste information, integrated with hunger and satiety, undergo a sensorimotor transformation to lead to the motor actions of feeding behavior. I examine what is known of feeding circuitry in adult flies from more than 250 years of work in larger flies and from newer work in Drosophila. I review the anatomy of the proboscis, its muscles and their functions (where known), its motor neurons, interneurons known to receive taste inputs, interneurons that diverge from taste circuitry to provide information to other circuits, interneurons from other circuits that converge on feeding circuits, proprioceptors that influence the motor control of feeding, and sites of integration of hunger and satiety on feeding circuits. In spite of the several neuron types now known, a connected pathway from taste inputs to feeding motor outputs has yet to be found. We are on the threshold of an era where these individual components will be assembled into circuits, revealing how nervous system architecture leads to the control of behavior.
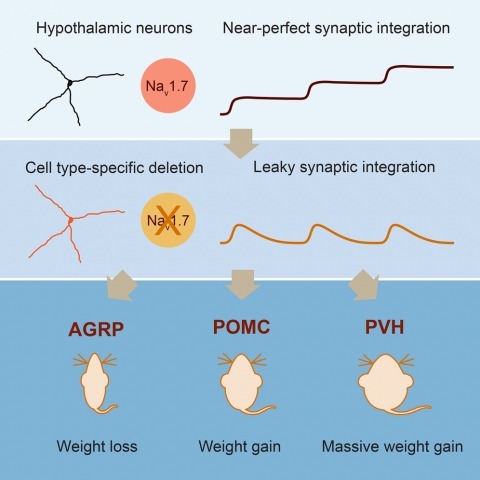
Neurons are well suited for computations on millisecond timescales, but some neuronal circuits set behavioral states over long time periods, such as those involved in energy homeostasis. We found that multiple types of hypothalamic neurons, including those that oppositely regulate body weight, are specialized as near-perfect synaptic integrators that summate inputs over extended timescales. Excitatory postsynaptic potentials (EPSPs) are greatly prolonged, outlasting the neuronal membrane time-constant up to 10-fold. This is due to the voltage-gated sodium channel Nav1.7 (Scn9a), previously associated with pain-sensation but not synaptic integration. Scn9a deletion in AGRP, POMC, or paraventricular hypothalamic neurons reduced EPSP duration, synaptic integration, and altered body weight in mice. In vivo whole-cell recordings in the hypothalamus confirmed near-perfect synaptic integration. These experiments show that integration of synaptic inputs over time by Nav1.7 is critical for body weight regulation and reveal a mechanism for synaptic control of circuits regulating long term homeostatic functions.