Filter
Associated Lab
Associated Project Team
Publication Date
Type of Publication
4 Publications
Showing 1-4 of 4 resultsThe present disclosure provides, inter alia, genetically encoded recombinant peptide biosensors comprising analyte-binding framework portions and signaling portions, wherein the signaling portions are present within the framework portions at sites or amino acid positions that undergo a conformational change upon interaction of the framework portion with an analyte.
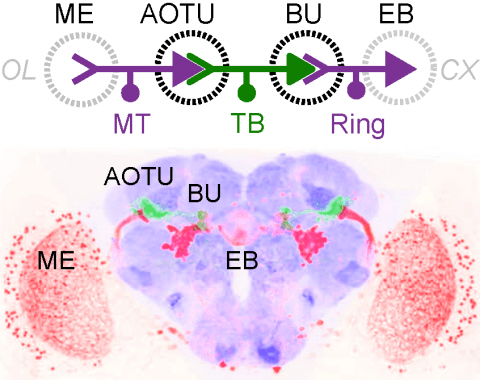
Many animals orient using visual cues, but how a single cue is selected from among many is poorly understood. Here we show that Drosophila ring neurons—central brain neurons implicated in navigation—display visual stimulus selection. Using in vivo two-color two-photon imaging with genetically encoded calcium indicators, we demonstrate that individual ring neurons inherit simple-cell-like receptive fields from their upstream partners. Stimuli in the contralateral visual field suppressed responses to ipsilateral stimuli in both populations. Suppression strength depended on when and where the contralateral stimulus was presented, an effect stronger in ring neurons than in their upstream inputs. This history-dependent effect on the temporal structure of visual responses, which was well modeled by a simple biphasic filter, may determine how visual references are selected for the fly's internal compass. Our approach highlights how two-color calcium imaging can help identify and localize the origins of sensory transformations across synaptically connected neural populations.
The calcium-modulated photoactivatable ratiometric integrator CaMPARI (Fosque et al., 2015) facilitates the study of neural circuits by permanently marking cells active during user-specified temporal windows. Permanent marking enables measurement of signals from large swathes of tissue and easy correlation of activity with other structural or functional labels. One potential application of CaMPARI is labeling neurons postsynaptic to specific populations targeted for optogenetic stimulation, giving rise to all-optical functional connectivity mapping. Here, we characterized the response of CaMPARI to several common types of neuronal calcium signals in mouse acute cortical brain slices. Our experiments show that CaMPARI is effectively converted by both action potentials and sub-threshold synaptic inputs, and that conversion level is correlated to synaptic strength. Importantly, we found that conversion rate can be tuned: it is linearly related to light intensity. At low photoconversion light levels CaMPARI offers a wide dynamic range due to slower conversion rate; at high light levels conversion is more rapid and more sensitive to activity. Finally, we employed CaMPARI and optogenetics for functional circuit mapping in ex vivo acute brain slices, which preserve in vivo-like connectivity of axon terminals. With a single light source, we stimulated channelrhodopsin-2-expressing long-range posteromedial (POm) thalamic axon terminals in cortex and induced CaMPARI conversion in recipient cortical neurons. We found that POm stimulation triggers robust photoconversion of layer 5 cortical neurons and weaker conversion of layer 2/3 neurons. Thus, CaMPARI enables network-wide, tunable, all-optical functional circuit mapping that captures supra- and sub-threshold depolarization. This article is protected by copyright. All rights reserved.
Although the endoplasmic reticulum (ER) extends throughout axons and axonal ER dysfunction is implicated in numerous neurological diseases, its role at nerve terminals is poorly understood. We developed novel genetically encoded ER-targeted low-affinity Ca(2+) indicators optimized for examining axonal ER Ca(2+). Our experiments revealed that presynaptic function is tightly controlled by ER Ca(2+) content. We found that neuronal activity drives net Ca(2+) uptake into presynaptic ER although this activity does not contribute significantly to shaping cytosolic Ca(2+) except during prolonged repetitive firing. In contrast, we found that axonal ER acts as an actuator of plasma membrane (PM) function: [Ca(2+)]ER controls STIM1 activation in presynaptic terminals, which results in the local modulation of presynaptic function, impacting activity-driven Ca(2+) entry and release probability. These experiments reveal a critical role of presynaptic ER in the control of neurotransmitter release and will help frame future investigations into the molecular basis of ER-driven neuronal disease states.