Filter
Associated Lab
- Aguilera Castrejon Lab (1) Apply Aguilera Castrejon Lab filter
- Ahrens Lab (45) Apply Ahrens Lab filter
- Aso Lab (39) Apply Aso Lab filter
- Baker Lab (19) Apply Baker Lab filter
- Betzig Lab (98) Apply Betzig Lab filter
- Beyene Lab (4) Apply Beyene Lab filter
- Bock Lab (14) Apply Bock Lab filter
- Branson Lab (45) Apply Branson Lab filter
- Card Lab (33) Apply Card Lab filter
- Cardona Lab (44) Apply Cardona Lab filter
- Chklovskii Lab (10) Apply Chklovskii Lab filter
- Clapham Lab (10) Apply Clapham Lab filter
- Cui Lab (19) Apply Cui Lab filter
- Darshan Lab (8) Apply Darshan Lab filter
- Dickson Lab (32) Apply Dickson Lab filter
- Druckmann Lab (21) Apply Druckmann Lab filter
- Dudman Lab (34) Apply Dudman Lab filter
- Eddy/Rivas Lab (30) Apply Eddy/Rivas Lab filter
- Egnor Lab (4) Apply Egnor Lab filter
- Espinosa Medina Lab (12) Apply Espinosa Medina Lab filter
- Feliciano Lab (6) Apply Feliciano Lab filter
- Fetter Lab (31) Apply Fetter Lab filter
- Fitzgerald Lab (15) Apply Fitzgerald Lab filter
- Freeman Lab (15) Apply Freeman Lab filter
- Funke Lab (34) Apply Funke Lab filter
- Gonen Lab (59) Apply Gonen Lab filter
- Grigorieff Lab (34) Apply Grigorieff Lab filter
- Harris Lab (48) Apply Harris Lab filter
- Heberlein Lab (13) Apply Heberlein Lab filter
- Hermundstad Lab (17) Apply Hermundstad Lab filter
- Hess Lab (66) Apply Hess Lab filter
- Ilanges Lab (1) Apply Ilanges Lab filter
- Jayaraman Lab (39) Apply Jayaraman Lab filter
- Ji Lab (33) Apply Ji Lab filter
- Johnson Lab (1) Apply Johnson Lab filter
- Karpova Lab (13) Apply Karpova Lab filter
- Keleman Lab (8) Apply Keleman Lab filter
- Keller Lab (60) Apply Keller Lab filter
- Lavis Lab (123) Apply Lavis Lab filter
- Lee (Albert) Lab (29) Apply Lee (Albert) Lab filter
- Leonardo Lab (19) Apply Leonardo Lab filter
- Li Lab (1) Apply Li Lab filter
- Lippincott-Schwartz Lab (87) Apply Lippincott-Schwartz Lab filter
- Liu (Zhe) Lab (53) Apply Liu (Zhe) Lab filter
- Looger Lab (136) Apply Looger Lab filter
- Magee Lab (31) Apply Magee Lab filter
- Menon Lab (12) Apply Menon Lab filter
- Murphy Lab (6) Apply Murphy Lab filter
- O'Shea Lab (3) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Pachitariu Lab (28) Apply Pachitariu Lab filter
- Pastalkova Lab (5) Apply Pastalkova Lab filter
- Pavlopoulos Lab (7) Apply Pavlopoulos Lab filter
- Pedram Lab (3) Apply Pedram Lab filter
- Podgorski Lab (16) Apply Podgorski Lab filter
- Reiser Lab (43) Apply Reiser Lab filter
- Riddiford Lab (20) Apply Riddiford Lab filter
- Romani Lab (28) Apply Romani Lab filter
- Rubin Lab (101) Apply Rubin Lab filter
- Saalfeld Lab (42) Apply Saalfeld Lab filter
- Satou Lab (1) Apply Satou Lab filter
- Scheffer Lab (36) Apply Scheffer Lab filter
- Schreiter Lab (44) Apply Schreiter Lab filter
- Shroff Lab (22) Apply Shroff Lab filter
- Simpson Lab (18) Apply Simpson Lab filter
- Singer Lab (37) Apply Singer Lab filter
- Spruston Lab (55) Apply Spruston Lab filter
- Stern Lab (69) Apply Stern Lab filter
- Sternson Lab (47) Apply Sternson Lab filter
- Stringer Lab (24) Apply Stringer Lab filter
- Svoboda Lab (131) Apply Svoboda Lab filter
- Tebo Lab (7) Apply Tebo Lab filter
- Tervo Lab (9) Apply Tervo Lab filter
- Tillberg Lab (14) Apply Tillberg Lab filter
- Tjian Lab (17) Apply Tjian Lab filter
- Truman Lab (58) Apply Truman Lab filter
- Turaga Lab (34) Apply Turaga Lab filter
- Turner Lab (24) Apply Turner Lab filter
- Vale Lab (6) Apply Vale Lab filter
- Voigts Lab (1) Apply Voigts Lab filter
- Wang (Meng) Lab (8) Apply Wang (Meng) Lab filter
- Wang (Shaohe) Lab (4) Apply Wang (Shaohe) Lab filter
- Wu Lab (8) Apply Wu Lab filter
- Zlatic Lab (26) Apply Zlatic Lab filter
- Zuker Lab (5) Apply Zuker Lab filter
Associated Project Team
- CellMap (1) Apply CellMap filter
- COSEM (3) Apply COSEM filter
- Fly Descending Interneuron (10) Apply Fly Descending Interneuron filter
- Fly Functional Connectome (14) Apply Fly Functional Connectome filter
- Fly Olympiad (5) Apply Fly Olympiad filter
- FlyEM (51) Apply FlyEM filter
- FlyLight (46) Apply FlyLight filter
- GENIE (40) Apply GENIE filter
- Integrative Imaging (1) Apply Integrative Imaging filter
- Larval Olympiad (2) Apply Larval Olympiad filter
- MouseLight (16) Apply MouseLight filter
- NeuroSeq (1) Apply NeuroSeq filter
- ThalamoSeq (1) Apply ThalamoSeq filter
- Tool Translation Team (T3) (24) Apply Tool Translation Team (T3) filter
- Transcription Imaging (45) Apply Transcription Imaging filter
Associated Support Team
- Anatomy and Histology (18) Apply Anatomy and Histology filter
- Cryo-Electron Microscopy (33) Apply Cryo-Electron Microscopy filter
- Electron Microscopy (12) Apply Electron Microscopy filter
- Fly Facility (39) Apply Fly Facility filter
- Gene Targeting and Transgenics (11) Apply Gene Targeting and Transgenics filter
- Integrative Imaging (10) Apply Integrative Imaging filter
- Janelia Experimental Technology (35) Apply Janelia Experimental Technology filter
- Management Team (1) Apply Management Team filter
- Molecular Genomics (15) Apply Molecular Genomics filter
- Primary & iPS Cell Culture (13) Apply Primary & iPS Cell Culture filter
- Project Technical Resources (34) Apply Project Technical Resources filter
- Quantitative Genomics (18) Apply Quantitative Genomics filter
- Scientific Computing Software (58) Apply Scientific Computing Software filter
- Scientific Computing Systems (6) Apply Scientific Computing Systems filter
- Viral Tools (14) Apply Viral Tools filter
- Vivarium (6) Apply Vivarium filter
Publication Date
- 2024 (124) Apply 2024 filter
- 2023 (175) Apply 2023 filter
- 2022 (166) Apply 2022 filter
- 2021 (174) Apply 2021 filter
- 2020 (177) Apply 2020 filter
- 2019 (177) Apply 2019 filter
- 2018 (206) Apply 2018 filter
- 2017 (186) Apply 2017 filter
- 2016 (191) Apply 2016 filter
- 2015 (195) Apply 2015 filter
- 2014 (190) Apply 2014 filter
- 2013 (136) Apply 2013 filter
- 2012 (112) Apply 2012 filter
- 2011 (98) Apply 2011 filter
- 2010 (61) Apply 2010 filter
- 2009 (56) Apply 2009 filter
- 2008 (40) Apply 2008 filter
- 2007 (21) Apply 2007 filter
- 2006 (3) Apply 2006 filter
2488 Janelia Publications
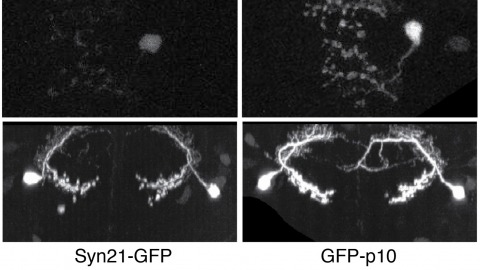
Showing 2411-2420 of 2488 resultsThe ability to specify the expression levels of exogenous genes inserted in the genomes of transgenic animals is critical for the success of a wide variety of experimental manipulations. Protein production can be regulated at the level of transcription, mRNA transport, mRNA half-life, or translation efficiency. In this report, we show that several well-characterized sequence elements derived from plant and insect viruses are able to function in Drosophila to increase the apparent translational efficiency of mRNAs by as much as 20-fold. These increases render expression levels sufficient for genetic constructs previously requiring multiple copies to be effective in single copy, including constructs expressing the temperature-sensitive inactivator of neuronal function Shibire(ts1), and for the use of cytoplasmic GFP to image the fine processes of neurons.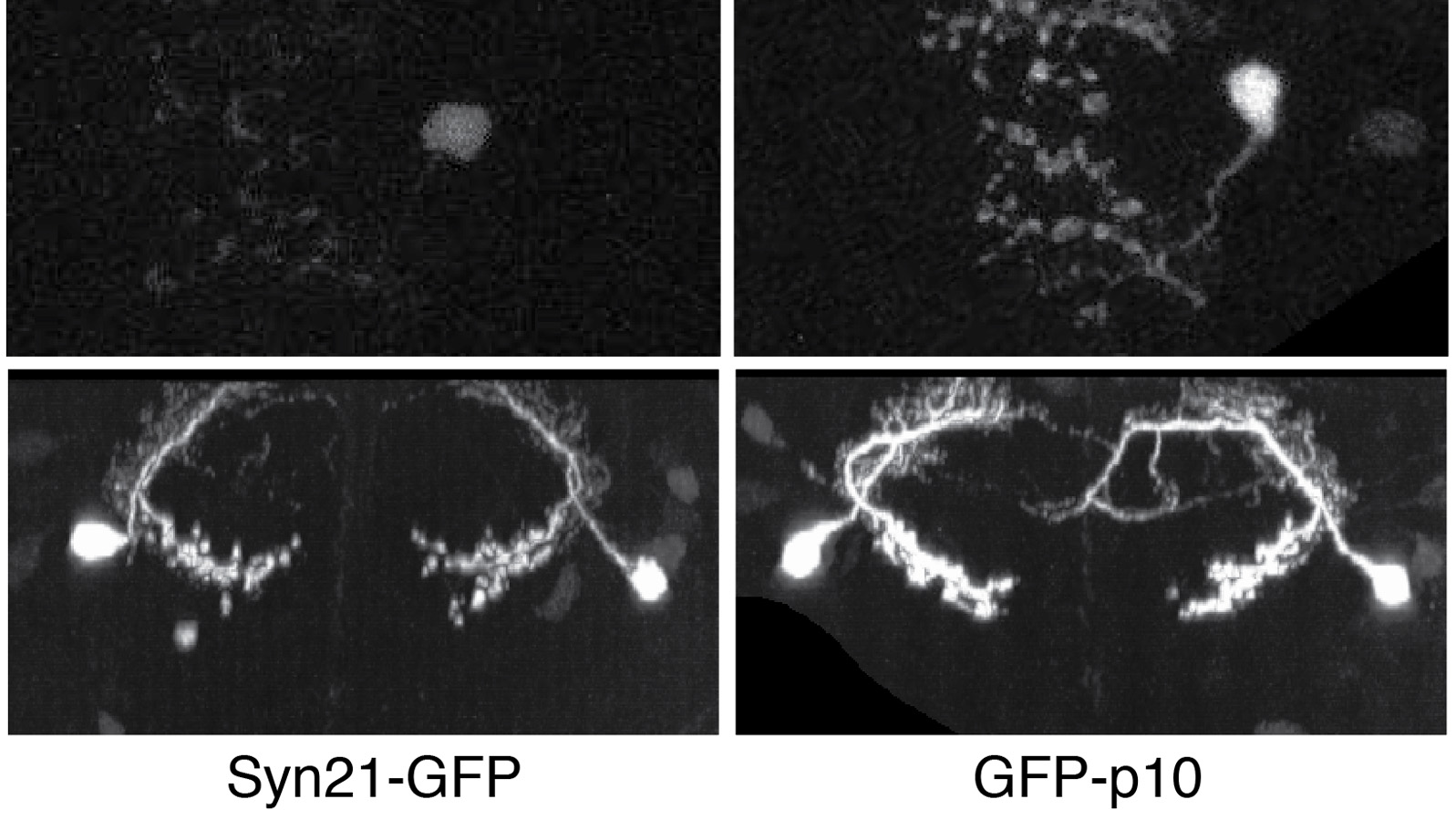
Capping Protein (CP) plays a central role in the creation of the Arp2/3-generated branched actin networks comprising lamellipodia and pseudopodia by virtue of its ability to cap the actin filament barbed end, which promotes Arp2/3-dependent filament nucleation and optimal branching. The highly conserved protein V-1/Myotrophin binds CP tightly in vitro to render it incapable of binding the barbed end. Here we addressed the physiological significance of this CP antagonist in Dictyostelium, which expresses a V-1 homolog that we show is very similar biochemically to mouse V-1. Consistent with previous studies of CP knockdown, overexpression of V-1 in Dictyostelium reduced the size of pseudopodia and the cortical content of Arp2/3 and induced the formation of filopodia. Importantly, these effects scaled positively with the degree of V-1 overexpression and were not seen with a V-1 mutant that cannot bind CP. V-1 is present in molar excess over CP, suggesting that it suppresses CP activity in the cytoplasm at steady state. Consistently, cells devoid of V-1, like cells overexpressing CP described previously, exhibited a significant decrease in cellular F-actin content. Moreover, V-1-null cells exhibited pronounced defects in macropinocytosis and chemotactic aggregation that were rescued by V-1, but not by the V-1 mutant. Together, these observations demonstrate that V-1 exerts significant influence in vivo on major actin-based processes via its ability to sequester CP. Finally, we present evidence that V-1's ability to sequester CP is regulated by phosphorylation, suggesting that cells may manipulate the level of active CP to tune their "actin phenotype."
The V3D system provides three-dimensional (3D) visualization of gigabyte-sized microscopy image stacks in real time on current laptops and desktops. V3D streamlines the online analysis, measurement and proofreading of complicated image patterns by combining ergonomic functions for selecting a location in an image directly in 3D space and for displaying biological measurements, such as from fluorescent probes, using the overlaid surface objects. V3D runs on all major computer platforms and can be enhanced by software plug-ins to address specific biological problems. To demonstrate this extensibility, we built a V3D-based application, V3D-Neuron, to reconstruct complex 3D neuronal structures from high-resolution brain images. V3D-Neuron can precisely digitize the morphology of a single neuron in a fruitfly brain in minutes, with about a 17-fold improvement in reliability and tenfold savings in time compared with other neuron reconstruction tools. Using V3D-Neuron, we demonstrate the feasibility of building a 3D digital atlas of neurite tracts in the fruitfly brain.
Visceral sensory pathways mediate homeostatic reflexes, the dysfunction of which leads to many neurological disorders. The Bezold-Jarisch reflex (BJR), first described in 1867, is a cardioinhibitory reflex that is speculated to be mediated by vagal sensory neurons (VSNs) that also triggers syncope. However, the molecular identity, anatomical organization, physiological characteristics and behavioural influence of cardiac VSNs remain mostly unknown. Here we leveraged single-cell RNA-sequencing data and HYBRiD tissue clearing to show that VSNs that express neuropeptide Y receptor Y2 (NPY2R) predominately connect the heart ventricular wall to the area postrema. Optogenetic activation of NPY2R VSNs elicits the classic triad of BJR responses-hypotension, bradycardia and suppressed respiration-and causes an animal to faint. Photostimulation during high-resolution echocardiography and laser Doppler flowmetry with behavioural observation revealed a range of phenotypes reflected in clinical syncope, including reduced cardiac output, cerebral hypoperfusion, pupil dilation and eye-roll. Large-scale Neuropixels brain recordings and machine-learning-based modelling showed that this manipulation causes the suppression of activity across a large distributed neuronal population that is not explained by changes in spontaneous behavioural movements. Additionally, bidirectional manipulation of the periventricular zone had a push-pull effect, with inhibition leading to longer syncope periods and activation inducing arousal. Finally, ablating NPY2R VSNs specifically abolished the BJR. Combined, these results demonstrate a genetically defined cardiac reflex that recapitulates characteristics of human syncope at physiological, behavioural and neural network levels.
Volume-object annotation system (VANO) is a cross-platform image annotation system that enables one to conveniently visualize and annotate 3D volume objects including nuclei and cells. An application of VANO typically starts with an initial collection of objects produced by a segmentation computation. The objects can then be labeled, categorized, deleted, added, split, merged and redefined. VANO has been used to build high-resolution digital atlases of the nuclei of Caenorhabditis elegans at the L1 stage and the nuclei of Drosophila melanogaster’s ventral nerve cord at the late embryonic stage. AVAILABILITY: Platform independent executables of VANO, a sample dataset, and a detailed description of both its design and usage are available at research.janelia.org/peng/proj/vano. VANO is open-source for co-development.
The hippocampal CA3 region is essential for pattern completion and generation of sharp-wave ripples. During these operations, coordinated activation of ensembles of CA3 pyramidal neurons produces spatiotemporally structured input patterns arriving onto dendrites of recurrently connected CA3 neurons. To understand how such input patterns are translated into specific output patterns, we characterized dendritic integration in CA3 pyramidal cells using two-photon imaging and glutamate uncaging. We found that thin dendrites of CA3 pyramidal neurons integrate synchronous synaptic input in a highly supralinear fashion. The amplification was primarily mediated by NMDA receptor activation and was present over a relatively broad range of spatiotemporal input patterns. The decay of voltage responses, temporal summation, and action potential output was regulated in a compartmentalized fashion mainly by a G-protein-activated inwardly rectifying K(+) current. Our results suggest that plastic dendritic integrative mechanisms may support ensemble behavior in pyramidal neurons of the hippocampal circuitry.
The conditional expression of hairpin constructs in Drosophila melanogaster has emerged in recent years as a method of choice in functional genomic studies. To date, upstream activating site-driven RNA interference constructs have been inserted into the genome randomly using P-element-mediated transformation, which can result in false negatives due to variable expression. To avoid this problem, we have developed a transgenic RNA interference vector based on the phiC31 site-specific integration method.
Linking activity in specific cell types with perception, cognition, and action, requires quantitative behavioral experiments in genetic model systems such as the mouse. In head-fixed primates, the combination of precise stimulus control, monitoring of motor output, and physiological recordings over large numbers of trials are the foundation on which many conceptually rich and quantitative studies have been built. Choice-based, quantitative behavioral paradigms for head-fixed mice have not been described previously. Here, we report a somatosensory absolute object localization task for head-fixed mice. Mice actively used their mystacial vibrissae (whiskers) to sense the location of a vertical pole presented to one side of the head and reported with licking whether the pole was in a target (go) or a distracter (no-go) location. Mice performed hundreds of trials with high performance (>90% correct) and localized to <0.95 mm (<6 degrees of azimuthal angle). Learning occurred over 1-2 weeks and was observed both within and across sessions. Mice could perform object localization with single whiskers. Silencing barrel cortex abolished performance to chance levels. We measured whisker movement and shape for thousands of trials. Mice moved their whiskers in a highly directed, asymmetric manner, focusing on the target location. Translation of the base of the whiskers along the face contributed substantially to whisker movements. Mice tended to maximize contact with the go (rewarded) stimulus while minimizing contact with the no-go stimulus. We conjecture that this may amplify differences in evoked neural activity between trial types.
Neurons and neural networks often extend hundreds of micrometers in three dimensions. Capturing the calcium transients associated with their activity requires volume imaging methods with subsecond temporal resolution. Such speed is a challenge for conventional two-photon laser-scanning microscopy, because it depends on serial focal scanning in 3D and indicators with limited brightness. Here we present an optical module that is easily integrated into standard two-photon laser-scanning microscopes to generate an axially elongated Bessel focus, which when scanned in 2D turns frame rate into volume rate. We demonstrated the power of this approach in enabling discoveries for neurobiology by imaging the calcium dynamics of volumes of neurons and synapses in fruit flies, zebrafish larvae, mice and ferrets in vivo. Calcium signals in objects as small as dendritic spines could be resolved at video rates, provided that the samples were sparsely labeled to limit overlap in their axially projected images.