Filter
Associated Lab
Associated Project Team
Publication Date
- Remove 2012-12-31 19:00 – 2013-12-31 19:00 filter 2012-12-31 19:00 – 2013-12-31 19:00
- March 28, 2013 (1) Apply March 28, 2013 filter
- March 26, 2013 (1) Apply March 26, 2013 filter
- March 22, 2013 (1) Apply March 22, 2013 filter
- March 12, 2013 (1) Apply March 12, 2013 filter
- March 6, 2013 (1) Apply March 6, 2013 filter
- March 4, 2013 (1) Apply March 4, 2013 filter
- March 1, 2013 (3) Apply March 1, 2013 filter
- Remove March 2013 filter March 2013
9 Janelia Publications
Showing 1-9 of 9 resultsObjective: While the contribution of α-Synuclein to neurodegeneration in Parkinson’s disease is well accepted, the putative impact of its close homologue, β-Synuclein, is enigmatic. β-Synuclein is widely expressed throughout the central nervous system as is α-Synuclein, but the physiological functions of both proteins remain unknown. Recent findings supported the view that β-Synuclein can act as an ameliorating regulator of α-Synuclein-induced neurotoxicity, having neuroprotective rather than neurodegenerative capabilities, and being non-aggregating due to absence of most part of the aggregation-promoting NAC domain. However, a mutation of β-Synuclein linked to dementia with Lewy bodies rendered the protein neurotoxic in transgenic mice and fibrillation of β-Synuclein has been demonstrated in vitro. Methods / Results: Supporting the hypothesis that β-Synuclein can act as a neurodegeneration-inducing factor we now demonstrate that wild-type β-Synuclein is neurotoxic for cultured primary neurons. Furthermore, β-Synuclein formed proteinase K resistant aggregates in dopaminergic neurons in vivo, leading to pronounced and progressive neurodegeneration in rats. Expression of β-Synuclein caused mitochondrial fragmentation, but this fragmentation did not render mitochondria non-functional in terms of ion handling and respiration even in late stages of neurodegeneration. A comparison of the neurodegenerative effects induced by α-, β-, and γ-Synuclein revealed that β-Synuclein was eventually as neurotoxic as α-Synuclein for nigral dopaminergic neurons, while γ-Synuclein proved to be non-toxic and had very low aggregation propensity. Interpretation: Our results suggest that the role of β-Synuclein as a putative modulator of neuropathology in aggregopathies like Parkinson’s disease and dementia with Lewy bodies needs to be revisited. ANN NEUROL 2013. © 2013 American Neurological Association.
Neuroscience is at a crossroads. Great effort is being invested into deciphering specific neural interactions and circuits. At the same time, there exist few general theories or principles that explain brain function. We attribute this disparity, in part, to limitations in current methodologies. Traditional neurophysiological approaches record the activities of one neuron or a few neurons at a time. Neurochemical approaches focus on single neurotransmitters. Yet, there is an increasing realization that neural circuits operate at emergent levels, where the interactions between hundreds or thousands of neurons, utilizing multiple chemical transmitters, generate functional states. Brains function at the nanoscale, so tools to study brains must ultimately operate at this scale, as well. Nanoscience and nanotechnology are poised to provide a rich toolkit of novel methods to explore brain function by enabling simultaneous measurement and manipulation of activity of thousands or even millions of neurons. We and others refer to this goal as the Brain Activity Mapping Project. In this Nano Focus, we discuss how recent developments in nanoscale analysis tools and in the design and synthesis of nanomaterials have generated optical, electrical, and chemical methods that can readily be adapted for use in neuroscience. These approaches represent exciting areas of technical development and research. Moreover, unique opportunities exist for nanoscientists, nanotechnologists, and other physical scientists and engineers to contribute to tackling the challenging problems involved in understanding the fundamentals of brain function.
Developmental signals such as Wnts are often presented to cells in an oriented manner. To examine the consequences of local Wnt signaling, we immobilized Wnt proteins on beads and introduced them to embryonic stem cells in culture. At the single-cell level, the Wnt-bead induced asymmetric distribution of Wnt-β-catenin signaling components, oriented the plane of mitotic division, and directed asymmetric inheritance of centrosomes. Before cytokinesis was completed, the Wnt-proximal daughter cell expressed high levels of nuclear β-catenin and pluripotency genes, whereas the distal daughter cell acquired hallmarks of differentiation. We suggest that a spatially restricted Wnt signal induces an oriented cell division that generates distinct cell fates at predictable positions relative to the Wnt source.
Automatic 3D digital reconstruction (tracing) of neurons embedded in noisy microscopic images is challenging, especially when the cell morphology is complex.
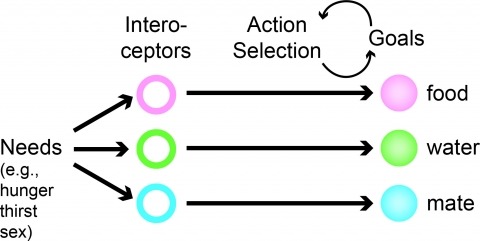
Neural processes that direct an animal’s actions toward environmental goals are critical elements for understanding behavior. The hypothalamus is closely associated with motivated behaviors required for survival and reproduction. Intense feeding, drinking, aggressive, and sexual behaviors can be produced by a simple neuronal stimulus applied to discrete hypothalamic regions. What can these "evoked behaviors" teach us about the neural processes that determine behavioral intent and intensity? Small populations of neurons sufficient to evoke a complex motivated behavior may be used as entry points to identify circuits that energize and direct behavior to specific goals. Here, I review recent applications of molecular genetic, optogenetic, and pharmacogenetic approaches that overcome previous limitations for analyzing anatomically complex hypothalamic circuits and their interactions with the rest of the brain. These new tools have the potential to bridge the gaps between neurobiological and psychological thinking about the mechanisms of complex motivated behavior.
Genetically encoded calcium indicators (GECIs) are powerful tools for systems neuroscience. Here we describe red, single-wavelength GECIs, "RCaMPs," engineered from circular permutation of the thermostable red fluorescent protein mRuby. High-resolution crystal structures of mRuby, the red sensor RCaMP, and the recently published red GECI R-GECO1 give insight into the chromophore environments of the Ca(2+)-bound state of the sensors and the engineered protein domain interfaces of the different indicators. We characterized the biophysical properties and performance of RCaMP sensors in vitro and in vivo in Caenorhabditis elegans, Drosophila larvae, and larval zebrafish. Further, we demonstrate 2-color calcium imaging both within the same cell (registering mitochondrial and somatic [Ca(2+)]) and between two populations of cells: neurons and astrocytes. Finally, we perform integrated optogenetics experiments, wherein neural activation via channelrhodopsin-2 (ChR2) or a red-shifted variant, and activity imaging via RCaMP or GCaMP, are conducted simultaneously, with the ChR2/RCaMP pair providing independently addressable spectral channels. Using this paradigm, we measure calcium responses of naturalistic and ChR2-evoked muscle contractions in vivo in crawling C. elegans. We systematically compare the RCaMP sensors to R-GECO1, in terms of action potential-evoked fluorescence increases in neurons, photobleaching, and photoswitching. R-GECO1 displays higher Ca(2+) affinity and larger dynamic range than RCaMP, but exhibits significant photoactivation with blue and green light, suggesting that integrated channelrhodopsin-based optogenetics using R-GECO1 may be subject to artifact. Finally, we create and test blue, cyan, and yellow variants engineered from GCaMP by rational design. This engineered set of chromatic variants facilitates new experiments in functional imaging and optogenetics.
Smart camera networks have recently emerged as a new class of sensor network infrastructure that is capable of supporting high-power in-network signal processing and enabling a wide range of applications. In this article, we provide an exposition of our efforts to build a low-bandwidth wireless camera network platform, called CITRIC, and its applications in smart camera networks. The platform integrates a camera, a microphone, a frequency-scalable (up to 624 MHz) CPU, 16 MB FLASH, and 64 MB RAM onto a single device. The device then connects with a standard sensor network mote to form a wireless camera mote. With reasonably low power consumption and extensive algorithmic libraries running on a decent operating system that is easy to program, CITRIC is ideal for research and applications in distributed image and video processing. Its capabilities of in-network image processing also reduce communication requirements, which has been high in other existing camera networks with centralized processing. Furthermore, the mote easily integrates with other low-bandwidth sensor networks via the IEEE 802.15.4 protocol. To justify the utility of CITRIC, we present several representative applications. In particular, concrete research results will be demonstrated in two areas, namely, distributed coverage hole identification and distributed object recognition.
Visualization of cellular and molecular processes is an indispensable tool for cell biologists, and innovations in microscopy methods unfailingly lead to new biological discoveries. Today, light microscopy (LM) provides ever-higher spatial and temporal resolution and visualization of biological process over enormous ranges. Electron microscopy (EM) is moving into the atomic resolution regime and allowing cellular analyses that are more physiological and sophisticated in scope. Importantly, much is being gained by combining multiple approaches, (e.g., LM and EM) to take advantage of their complementary strengths. The advent of high-throughput microscopies has led to a common need for sophisticated computational methods to quantitatively analyze huge amounts of data and translate images into new biological insights.
We are interested in establishing the correspondence between neuron activity and body curvature during various movements of C. Elegans worms. Given long sequences of images, specifically recorded to glow when the neuron is active, it is required to track all identifiable neurons in each frame. The characteristics of the neuron data, e.g., the uninformative nature of neuron appearance and the sequential ordering of neurons, renders standard single and multi-object tracking methods either ineffective or unnecessary for our task. In this paper, we propose a multi-target tracking algorithm that correctly assigns each neuron to one of several candidate locations in the next frame preserving shape constraint. The results demonstrate how the proposed method can robustly track more neurons than several existing methods in long sequences of images.