Filter
Associated Lab
5 Janelia Publications
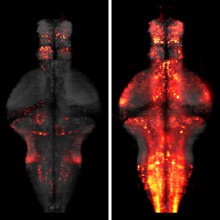
Showing 1-5 of 5 resultsWhole-brain imaging allows for comprehensive functional mapping of distributed neural pathways, but neuronal perturbation experiments are usually limited to targeting predefined regions or genetically identifiable cell types. To complement whole-brain measures of activity with brain-wide manipulations for testing causal interactions, we introduce a system that uses measuredactivity patterns to guide optical perturbations of any subset of neurons in the same fictively behaving larval zebrafish. First, a light-sheet microscope collects whole-brain data that are rapidly analyzed by a distributed computing system to generate functional brain maps. On the basis of these maps, the experimenter can then optically ablate neurons and image activity changes across the brain. We applied this method to characterize contributions of behaviorally tuned populations to the optomotor response. We extended the system to optogenetically stimulate arbitrary subsets of neurons during whole-brain imaging. These open-source methods enable delineating the contributions of neurons to brain-wide circuit dynamics and behavior in individual animals.
The nature of nervous system function and development is inherently global, since all components eventually influence one another. Networks communicate through dense synaptic, electric, and modulatory connections and develop through concurrent growth and interlinking of their neurons, processes, glia, and blood vessels. These factors drive the development of techniques capable of imaging neural signaling, anatomy, and developmental processes at ever-larger scales. Here, we discuss the nature of questions benefitting from large-scale imaging techniques and introduce recent applications. We focus on emerging light-sheet microscopy approaches, which are well suited for live imaging of large systems with high spatiotemporal resolution and over long periods of time. We also discuss computational methods suitable for extracting biological information from the resulting system-level image data sets. Together with new tools for reporting and manipulating neuronal activity and gene expression, these techniques promise new insights into the large-scale function and development of neural systems.
Developments in electrical and optical recording technology are scaling up the size of neuronal populations that can be monitored simultaneously. Light-sheet imaging is rapidly gaining traction as a method for optically interrogating activity in large networks and presents both opportunities and challenges for understanding circuit function.
The processing of sensory input and the generation of behavior involves large networks of neurons, which necessitates new technology for recording from many neurons in behaving animals. In the larval zebrafish, light-sheet microscopy can be used to record the activity of almost all neurons in the brain simultaneously at single-cell resolution. Existing implementations, however, cannot be combined with visually driven behavior because the light sheet scans over the eye, interfering with presentation of controlled visual stimuli. Here we describe a system that overcomes the confounding eye stimulation through the use of two light sheets and combines whole-brain light-sheet imaging with virtual reality for fictively behaving larval zebrafish.
Brain function relies on communication between large populations of neurons across multiple brain areas, a full understanding of which would require knowledge of the time-varying activity of all neurons in the central nervous system. Here we use light-sheet microscopy to record activity, reported through the genetically encoded calcium indicator GCaMP5G, from the entire volume of the brain of the larval zebrafish in vivo at 0.8 Hz, capturing more than 80% of all neurons at single-cell resolution. Demonstrating how this technique can be used to reveal functionally defined circuits across the brain, we identify two populations of neurons with correlated activity patterns. One circuit consists of hindbrain neurons functionally coupled to spinal cord neuropil. The other consists of an anatomically symmetric population in the anterior hindbrain, with activity in the left and right halves oscillating in antiphase, on a timescale of 20 s, and coupled to equally slow oscillations in the inferior olive.