Filter
Associated Lab
- Dudman Lab (1) Apply Dudman Lab filter
- Hermundstad Lab (1) Apply Hermundstad Lab filter
- Lavis Lab (1) Apply Lavis Lab filter
- Lee (Albert) Lab (1) Apply Lee (Albert) Lab filter
- Looger Lab (2) Apply Looger Lab filter
- Pachitariu Lab (1) Apply Pachitariu Lab filter
- Saalfeld Lab (2) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Remove Sternson Lab filter Sternson Lab
- Svoboda Lab (4) Apply Svoboda Lab filter
- Tillberg Lab (3) Apply Tillberg Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
Associated Project Team
Publication Date
- 2023 (2) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (3) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (2) Apply 2019 filter
- 2017 (2) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (7) Apply 2015 filter
- 2014 (5) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (4) Apply 2012 filter
- 2011 (6) Apply 2011 filter
- 2010 (1) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2008 (1) Apply 2008 filter
- 2005 (1) Apply 2005 filter
- 2004 (1) Apply 2004 filter
- 2002 (1) Apply 2002 filter
- 2001 (2) Apply 2001 filter
- 1998 (1) Apply 1998 filter
Type of Publication
54 Publications
Showing 31-40 of 54 resultsNeural processes that direct an animal’s actions toward environmental goals are critical elements for understanding behavior. The hypothalamus is closely associated with motivated behaviors required for survival and reproduction. Intense feeding, drinking, aggressive, and sexual behaviors can be produced by a simple neuronal stimulus applied to discrete hypothalamic regions. What can these "evoked behaviors" teach us about the neural processes that determine behavioral intent and intensity? Small populations of neurons sufficient to evoke a complex motivated behavior may be used as entry points to identify circuits that energize and direct behavior to specific goals. Here, I review recent applications of molecular genetic, optogenetic, and pharmacogenetic approaches that overcome previous limitations for analyzing anatomically complex hypothalamic circuits and their interactions with the rest of the brain. These new tools have the potential to bridge the gaps between neurobiological and psychological thinking about the mechanisms of complex motivated behavior.
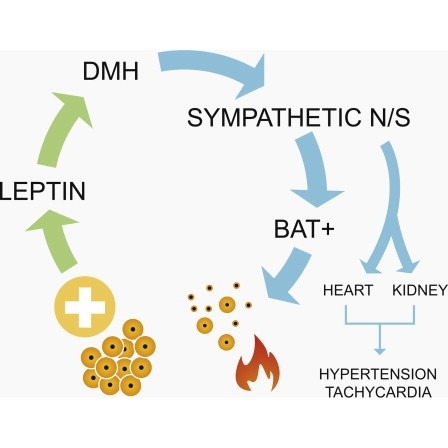
Obesity is associated with increased blood pressure (BP), which in turn increases the risk of cardiovascular diseases. We found that the increase in leptin levels seen in diet-induced obesity (DIO) drives an increase in BP in rodents, an effect that was not seen in animals deficient in leptin or leptin receptors (LepR). Furthermore, humans with loss-of-function mutations in leptin and the LepR have low BP despite severe obesity. Leptin's effects on BP are mediated by neuronal circuits in the dorsomedial hypothalamus (DMH), as blocking leptin with a specific antibody, antagonist, or inhibition of the activity of LepR-expressing neurons in the DMH caused a rapid reduction of BP in DIO mice, independent of changes in weight. Re-expression of LepRs in the DMH of DIO LepR-deficient mice caused an increase in BP. These studies demonstrate that leptin couples changes in weight to changes in BP in mammalian species.
The central actions of leptin are essential for homeostatic control of adipose tissue mass, glucose metabolism, and many autonomic and neuroendocrine systems. In the brain, leptin acts on numerous different cell types via the long-form leptin receptor (LepRb) to elicit its effects. The precise identification of leptin’s cellular targets is fundamental to understanding the mechanism of its pleiotropic central actions. We have systematically characterized LepRb distribution in the mouse brain using in situ hybridization in wildtype mice as well as by EYFP immunoreactivity in a novel LepRb-IRES-Cre EYFP reporter mouse line showing high levels of LepRb mRNA/EYFP coexpression. We found substantial LepRb mRNA and EYFP expression in hypothalamic and extrahypothalamic sites described before, including the dorsomedial nucleus of the hypothalamus, ventral premammillary nucleus, ventral tegmental area, parabrachial nucleus, and the dorsal vagal complex. Expression in insular cortex, lateral septal nucleus, medial preoptic area, rostral linear nucleus, and in the Edinger-Westphal nucleus was also observed and had been previously unreported. The LepRb-IRES-Cre reporter line was used to chemically characterize a population of leptin receptor-expressing neurons in the midbrain. Tyrosine hydroxylase and Cre reporter were found to be coexpressed in the ventral tegmental area and in other midbrain dopaminergic neurons. Lastly, the LepRb-IRES-Cre reporter line was used to map the extent of peripheral leptin sensing by central nervous system (CNS) LepRb neurons. Thus, we provide data supporting the use of the LepRb-IRES-Cre line for the assessment of the anatomic and functional characteristics of neurons expressing leptin receptor.
A specialist neuron uses an intriguing process to help control the body's response to hunger. A lipid pathway involving the breakdown of cellular components regulates the expression of a neuropeptide that affects feeding and body weight.
Modular synthesis and substrate stereocontrol were combined to furnish 18,000 diverse 1,3-dioxanes whose distribution in chemical space rivals that of a reference set of over 2,000 bioactive small molecules. Library quality was assessed at key synthetic stages, culminating in a detailed postsynthesis analysis of purity, yield, and structural characterizability, and the resynthesis of library subsets that did not meet quality standards. The importance of this analysis-resynthesis process is highlighted by the discovery of new biological probes through organismal and protein binding assays, and by determination of the building block and stereochemical basis for their bioactivity. This evaluation of a portion of the 1,3-dioxane library suggests that many additional probes for chemical genetics will be identified as the entire library becomes biologically annotated.
Primary aldosteronism (PA) is the most frequent form of secondary hypertension. Over the past two decades, major advances have been made in our understanding of the disease with the identification of germline or somatic mutations in ion channels and pumps. These mutations enhance calcium signaling, the main trigger of aldosterone biosynthesis.
The central nucleus of the amygdala (CEA) is a brain region that integrates external and internal sensory information and executes innate and adaptive behaviors through distinct output pathways. Despite its complex functions, the diversity of molecularly defined neuronal types in the CEA and their contributions to major axonal projection targets have not been examined systematically. Here, we performed single-cell RNA-sequencing (scRNA-Seq) to classify molecularly defined cell types in the CEA and identified marker-genes to map the location of these neuronal types using expansion assisted iterative fluorescence in situ hybridization (EASI-FISH). We developed new methods to integrate EASI-FISH with 5-plex retrograde axonal labeling to determine the spatial, morphological, and connectivity properties of ∼30,000 molecularly defined CEA neurons. Our study revealed spatio-molecular organization of the CEA, with medial and lateral CEA associated with distinct cell families. We also found a long-range axon projection network from the CEA, where target regions receive inputs from multiple molecularly defined cell types. Axon collateralization was found primarily among projections to hindbrain targets, which are distinct from forebrain projections. This resource reports marker-gene combinations for molecularly defined cell types and axon-projection types, which will be useful for selective interrogation of these neuronal populations to study their contributions to the diverse functions of the CEA.
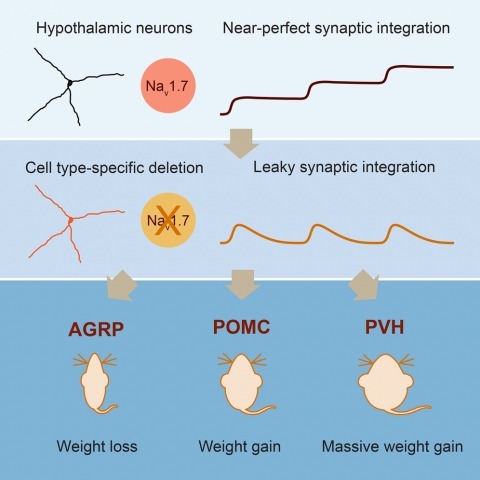
Neurons are well suited for computations on millisecond timescales, but some neuronal circuits set behavioral states over long time periods, such as those involved in energy homeostasis. We found that multiple types of hypothalamic neurons, including those that oppositely regulate body weight, are specialized as near-perfect synaptic integrators that summate inputs over extended timescales. Excitatory postsynaptic potentials (EPSPs) are greatly prolonged, outlasting the neuronal membrane time-constant up to 10-fold. This is due to the voltage-gated sodium channel Nav1.7 (Scn9a), previously associated with pain-sensation but not synaptic integration. Scn9a deletion in AGRP, POMC, or paraventricular hypothalamic neurons reduced EPSP duration, synaptic integration, and altered body weight in mice. In vivo whole-cell recordings in the hypothalamus confirmed near-perfect synaptic integration. These experiments show that integration of synaptic inputs over time by Nav1.7 is critical for body weight regulation and reveal a mechanism for synaptic control of circuits regulating long term homeostatic functions.
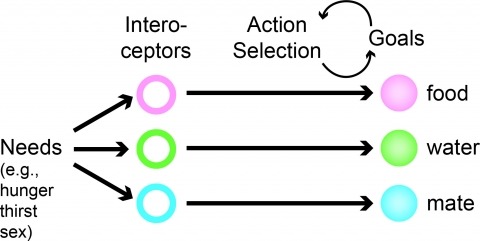
How does an organism’s internal state direct its actions? At one moment an animal forages for food with acrobatic feats such as tree climbing and jumping between branches. At another time, it travels along the ground to find water or a mate, exposing itself to predators along the way. These behaviors are costly in terms of energy or physical risk, and the likelihood of performing one set of actions relative to another is strongly modulated by internal state. For example, an animal in energy deficit searches for food and a dehydrated animal looks for water. The crosstalk between physiological state and motivational processes influences behavioral intensity and intent, but the underlying neural circuits are poorly understood. Molecular genetics along with optogenetic and pharmacogenetic tools for perturbing neuron function have enabled cell type-selective dissection of circuits that mediate behavioral responses to physiological state changes. Here, we review recent progress into neural circuit analysis of hunger in the mouse by focusing on a starvation-sensitive neuron population in the hypothalamus that is sufficient to promote voracious eating. We also consider research into the motivational processes that are thought to underlie hunger in order to outline considerations for bridging the gap between homeostatic and motivational neural circuits.
Mice lacking leptin receptors are grossly obese and diabetic, in part due to dysfunction in brain circuits important for energy homeostasis. Transplantation of leptin receptor-expressing hypothalamic progenitor neurons into the brains of leptin receptor deficient mice led to integration into neural circuits, reduced obesity, and normalized circulating glucose levels.