Filter
Associated Lab
- Aso Lab (30) Apply Aso Lab filter
- Betzig Lab (1) Apply Betzig Lab filter
- Bock Lab (2) Apply Bock Lab filter
- Branson Lab (8) Apply Branson Lab filter
- Card Lab (5) Apply Card Lab filter
- Clapham Lab (1) Apply Clapham Lab filter
- Dickson Lab (2) Apply Dickson Lab filter
- Druckmann Lab (1) Apply Druckmann Lab filter
- Fetter Lab (1) Apply Fetter Lab filter
- Funke Lab (1) Apply Funke Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Heberlein Lab (2) Apply Heberlein Lab filter
- Hermundstad Lab (2) Apply Hermundstad Lab filter
- Hess Lab (5) Apply Hess Lab filter
- Jayaraman Lab (6) Apply Jayaraman Lab filter
- Lippincott-Schwartz Lab (1) Apply Lippincott-Schwartz Lab filter
- Looger Lab (2) Apply Looger Lab filter
- O'Shea Lab (1) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Reiser Lab (15) Apply Reiser Lab filter
- Riddiford Lab (1) Apply Riddiford Lab filter
- Romani Lab (1) Apply Romani Lab filter
- Rubin Lab (143) Apply Rubin Lab filter
- Saalfeld Lab (4) Apply Saalfeld Lab filter
- Scheffer Lab (7) Apply Scheffer Lab filter
- Schreiter Lab (1) Apply Schreiter Lab filter
- Simpson Lab (3) Apply Simpson Lab filter
- Singer Lab (1) Apply Singer Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Stern Lab (1) Apply Stern Lab filter
- Svoboda Lab (3) Apply Svoboda Lab filter
- Tjian Lab (1) Apply Tjian Lab filter
- Truman Lab (4) Apply Truman Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
- Turner Lab (5) Apply Turner Lab filter
- Zuker Lab (1) Apply Zuker Lab filter
Associated Project Team
- CellMap (1) Apply CellMap filter
- Fly Functional Connectome (4) Apply Fly Functional Connectome filter
- Fly Olympiad (3) Apply Fly Olympiad filter
- FlyEM (11) Apply FlyEM filter
- FlyLight (20) Apply FlyLight filter
- GENIE (1) Apply GENIE filter
- Transcription Imaging (1) Apply Transcription Imaging filter
Publication Date
- 2025 (4) Apply 2025 filter
- 2024 (4) Apply 2024 filter
- 2023 (5) Apply 2023 filter
- 2022 (1) Apply 2022 filter
- 2021 (4) Apply 2021 filter
- 2020 (9) Apply 2020 filter
- 2019 (6) Apply 2019 filter
- 2018 (7) Apply 2018 filter
- 2017 (15) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (16) Apply 2015 filter
- 2014 (9) Apply 2014 filter
- 2013 (5) Apply 2013 filter
- 2012 (8) Apply 2012 filter
- 2011 (4) Apply 2011 filter
- 2010 (4) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2008 (4) Apply 2008 filter
- 2007 (2) Apply 2007 filter
- 2006 (1) Apply 2006 filter
- 2002 (1) Apply 2002 filter
- 2000 (2) Apply 2000 filter
- 1999 (1) Apply 1999 filter
- 1997 (1) Apply 1997 filter
- 1995 (2) Apply 1995 filter
- 1994 (2) Apply 1994 filter
- 1993 (2) Apply 1993 filter
- 1992 (1) Apply 1992 filter
- 1991 (2) Apply 1991 filter
- 1990 (3) Apply 1990 filter
- 1989 (2) Apply 1989 filter
- 1987 (2) Apply 1987 filter
- 1986 (1) Apply 1986 filter
- 1985 (1) Apply 1985 filter
- 1984 (1) Apply 1984 filter
- 1983 (1) Apply 1983 filter
- 1982 (2) Apply 1982 filter
- 1981 (1) Apply 1981 filter
- 1979 (1) Apply 1979 filter
- 1973 (1) Apply 1973 filter
Type of Publication
143 Publications
Showing 101-110 of 143 resultsDopamine signals reward in animal brains. A single presentation of a sugar reward to Drosophila activates distinct subsets of dopamine neurons that independently induce short- and long-term olfactory memories (STM and LTM, respectively). In this study, we show that a recurrent reward circuit underlies the formation and consolidation of LTM. This feedback circuit is composed of a single class of reward-signaling dopamine neurons (PAM-α1) projecting to a restricted region of the mushroom body (MB), and a specific MB output cell type, MBON-α1, whose dendrites arborize that same MB compartment. Both MBON-α1 and PAM-α1 neurons are required during the acquisition and consolidation of appetitive LTM. MBON-α1 additionally mediates the retrieval of LTM, which is dependent on the dopamine receptor signaling in the MB α/β neurons. Our results suggest that a reward signal transforms a nascent memory trace into a stable LTM using a feedback circuit at the cost of memory specificity.
The determination of cell fates during the assembly of the ommatidia in the compound eye of Drosophila appears to be controlled by cell-cell interactions. In this process, the sevenless gene is essential for the development of a single type of photoreceptor cell. In the absence of proper sevenless function the cells that would normally become the R7 photoreceptors instead become nonneuronal cells. Previous morphological and genetic analysis has indicated that the product of the sevenless gene is involved in reading or interpreting the positional information that specifies this particular developmental pathway. The sevenless gene has now been isolated and characterized. The data indicate that sevenless encodes a transmembrane protein with a tyrosine kinase domain. This structural similarity between sevenless and certain hormone receptors suggests that similar mechanisms are involved in developmental decisions based on cell-cell interaction and physiological or developmental changes induced by diffusible factors.
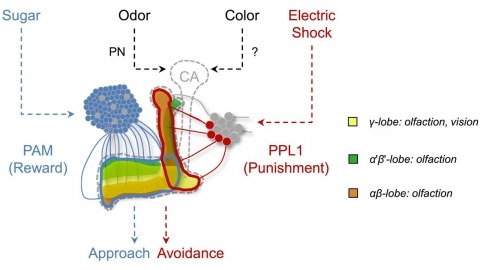
In nature, animals form memories associating reward or punishment with stimuli from different sensory modalities, such as smells and colors. It is unclear, however, how distinct sensory memories are processed in the brain. We established appetitive and aversive visual learning assays for Drosophila that are comparable to the widely used olfactory learning assays. These assays share critical features, such as reinforcing stimuli (sugar reward and electric shock punishment), and allow direct comparison of the cellular requirements for visual and olfactory memories. We found that the same subsets of dopamine neurons drive formation of both sensory memories. Furthermore, distinct yet partially overlapping subsets of mushroom body intrinsic neurons are required for visual and olfactory memories. Thus, our results suggest that distinct sensory memories are processed in a common brain center. Such centralization of related brain functions is an economical design that avoids the repetition of similar circuit motifs.
Animals rely on visual motion for navigating the world, and research in flies has clarified how neural circuits extract information from moving visual scenes. However, the major pathways connecting these patterns of optic flow to behavior remain poorly understood. Using a high-throughput quantitative assay of visually guided behaviors and genetic neuronal silencing, we discovered a region in Drosophila’s protocerebrum critical for visual motion following. We used neuronal silencing, calcium imaging, and optogenetics to identify a single cell type, LPC1, that innervates this region, detects translational optic flow, and plays a key role in regulating forward walking. Moreover, the population of LPC1s can estimate the travelling direction, such as when gaze direction diverges from body heading. By linking specific cell types and their visual computations to specific behaviors, our findings establish a foundation for understanding how the nervous system uses vision to guide navigation.
Animals are often bombarded with visual information and must prioritize specific visual features based on their current needs. The neuronal circuits that detect and relay visual features have been well studied. Much less is known about how an animal adjusts its visual attention as its goals or environmental conditions change. During social behaviours, flies need to focus on nearby flies. Here we study how the flow of visual information is altered when female Drosophila enter an aggressive state. From the connectome, we identify three state-dependent circuit motifs poised to modify the response of an aggressive female to fly-sized visual objects: convergence of excitatory inputs from neurons conveying select visual features and internal state; dendritic disinhibition of select visual feature detectors; and a switch that toggles between two visual feature detectors. Using cell-type-specific genetic tools, together with behavioural and neurophysiological analyses, we show that each of these circuit motifs is used during female aggression. We reveal that features of this same switch operate in male Drosophila during courtship pursuit, suggesting that disparate social behaviours may share circuit mechanisms. Our study provides a compelling example of using the connectome to infer circuit mechanisms that underlie dynamic processing of sensory signals.
In the development of multicellular organisms a diversity of cell types differentiate at specific positions. Spacing patterns, in which an array of two or more cell types forms from a uniform field of cells, are a common feature of development. Identical precursor cells may adopt different fates because of competition and inhibition between them. Such a pattern in the developing Drosophila eye is the evenly spaced array of R8 cells, around which other cell types are subsequently recruited. Genetic studies suggest that the scabrous mutation disrupts a signal produced by R8 cells that inhibits other cells from also becoming R8 cells. The scabrous locus was cloned, and it appears to encode a secreted protein partly related to the beta and gamma chains of fibrinogen. It is proposed that the sca locus encodes a lateral inhibitor of R8 differentiation. The roles of the Drosophila EGF-receptor homologue (DER) and Notch genes in this process were also investigated.
Visual systems can exploit spatial correlations in the visual scene by using retinotopy. However, retinotopy is often lost, such as when visual pathways are integrated with other sensory modalities. How is spatial information processed outside of strictly visual brain areas? Here, we focused on visual looming responsive LC6 cells in , a population whose dendrites collectively cover the visual field, but whose axons form a single glomerulus-a structure without obvious retinotopic organization-in the central brain. We identified multiple cell types downstream of LC6 in the glomerulus and found that they more strongly respond to looming in different portions of the visual field, unexpectedly preserving spatial information. Through EM reconstruction of all LC6 synaptic inputs to the glomerulus, we found that LC6 and downstream cell types form circuits within the glomerulus that enable spatial readout of visual features and contralateral suppression-mechanisms that transform visual information for behavioral control.
Building a sizable, complex brain requires both cellular expansion and diversification. One mechanism to achieve these goals is production of multiple transiently amplifying intermediate neural progenitors (INPs) from a single neural stem cell. Like mammalian neural stem cells, Drosophila type II neuroblasts utilize INPs to produce neurons and glia. Within a given lineage, the consecutively born INPs produce morphologically distinct progeny, presumably due to differential inheritance of temporal factors. To uncover the underlying temporal fating mechanisms, we profiled type II neuroblasts' transcriptome across time. Our results reveal opposing temporal gradients of Imp and Syp RNA-binding proteins (descending and ascending, respectively). Maintaining high Imp throughout serial INP production expands the number of neurons and glia with early temporal fate at the expense of cells with late fate. Conversely, precocious upregulation of Syp reduces the number of cells with early fate. Furthermore, we reveal that the transcription factor Seven-up initiates progression of the Imp/Syp gradients. Interestingly, neuroblasts that maintain initial Imp/Syp levels can still yield progeny with a small range of early fates. We therefore propose that the Seven-up-initiated Imp/Syp gradients create coarse temporal windows within type II neuroblasts to pattern INPs, which subsequently undergo fine-tuned subtemporal patterning.
Color and polarization provide complementary information about the world and are detected by specialized photoreceptors. However, the downstream neural circuits that process these distinct modalities are incompletely understood in any animal. Using electron microscopy, we have systematically reconstructed the synaptic targets of the photoreceptors specialized to detect color and skylight polarization in Drosophila, and we have used light microscopy to confirm many of our findings. We identified known and novel downstream targets that are selective for different wavelengths or polarized light, and followed their projections to other areas in the optic lobes and the central brain. Our results revealed many synapses along the photoreceptor axons between brain regions, new pathways in the optic lobes, and spatially segregated projections to central brain regions. Strikingly, photoreceptors in the polarization-sensitive dorsal rim area target fewer cell types, and lack strong connections to the lobula, a neuropil involved in color processing. Our reconstruction identifies shared wiring and modality-specific specializations for color and polarization vision, and provides a comprehensive view of the first steps of the pathways processing color and polarized light inputs.
Neuronal connectivity in the circadian clock network is essential for robust endogenous timekeeping. In the Drosophila circadian clock network, the four pairs of small ventral lateral neurons (sLNvs) serve as main pacemakers. Peptidergic communication via sLNv, which release the key output neuropeptide, Pigment Dispersing Factor (PDF), has been well characterized. In the absence of PDF, flies become largely arrhythmic, similar to the phenotype associated with the loss of the mammalian circadian peptide, VIP. In contrast, little is known about the role of the synaptic connections that sLNvs form with downstream neurons. Connectomic analyses revealed that despite their role as key pacemaker neurons within the clock network, the sLNvs form few connections with other clock neurons. However, they form strong synaptic connections with a small group of previously uncharacterized neurons, SLP316, which in turn synapse onto dorsal clock neurons. Here, we show that silencing SLP316 neurons via tetanus toxin (TNT) expression shortens the free-running period, whereas hyper-exciting them by expressing the constitutively open Na[+] channel, NaChBac, results in period lengthening. Under light-dark cycles, silencing SLP316 neurons also causes lower daytime activity and higher daytime sleep. Our results revealed that the main postsynaptic partners of the Drosophila pacemaker neurons are a non-clock neuronal cell type that regulates the timing of sleep and activity.