Filter
Associated Lab
- Aso Lab (29) Apply Aso Lab filter
- Betzig Lab (1) Apply Betzig Lab filter
- Bock Lab (2) Apply Bock Lab filter
- Branson Lab (7) Apply Branson Lab filter
- Card Lab (5) Apply Card Lab filter
- Clapham Lab (1) Apply Clapham Lab filter
- Dickson Lab (2) Apply Dickson Lab filter
- Druckmann Lab (1) Apply Druckmann Lab filter
- Fetter Lab (1) Apply Fetter Lab filter
- Funke Lab (1) Apply Funke Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Heberlein Lab (2) Apply Heberlein Lab filter
- Hermundstad Lab (2) Apply Hermundstad Lab filter
- Hess Lab (5) Apply Hess Lab filter
- Jayaraman Lab (5) Apply Jayaraman Lab filter
- Lippincott-Schwartz Lab (1) Apply Lippincott-Schwartz Lab filter
- Looger Lab (2) Apply Looger Lab filter
- O'Shea Lab (1) Apply O'Shea Lab filter
- Otopalik Lab (1) Apply Otopalik Lab filter
- Reiser Lab (15) Apply Reiser Lab filter
- Riddiford Lab (1) Apply Riddiford Lab filter
- Romani Lab (1) Apply Romani Lab filter
- Remove Rubin Lab filter Rubin Lab
- Saalfeld Lab (4) Apply Saalfeld Lab filter
- Scheffer Lab (7) Apply Scheffer Lab filter
- Schreiter Lab (1) Apply Schreiter Lab filter
- Simpson Lab (3) Apply Simpson Lab filter
- Singer Lab (1) Apply Singer Lab filter
- Spruston Lab (1) Apply Spruston Lab filter
- Stern Lab (1) Apply Stern Lab filter
- Svoboda Lab (3) Apply Svoboda Lab filter
- Tjian Lab (1) Apply Tjian Lab filter
- Truman Lab (4) Apply Truman Lab filter
- Turaga Lab (1) Apply Turaga Lab filter
- Turner Lab (5) Apply Turner Lab filter
- Zuker Lab (1) Apply Zuker Lab filter
Associated Project Team
Publication Date
- 2024 (3) Apply 2024 filter
- 2023 (6) Apply 2023 filter
- 2022 (1) Apply 2022 filter
- 2021 (4) Apply 2021 filter
- 2020 (9) Apply 2020 filter
- 2019 (6) Apply 2019 filter
- 2018 (7) Apply 2018 filter
- 2017 (15) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (16) Apply 2015 filter
- 2014 (9) Apply 2014 filter
- 2013 (5) Apply 2013 filter
- 2012 (8) Apply 2012 filter
- 2011 (4) Apply 2011 filter
- 2010 (4) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2008 (4) Apply 2008 filter
- 2007 (2) Apply 2007 filter
- 2006 (1) Apply 2006 filter
- 2002 (1) Apply 2002 filter
- 2000 (2) Apply 2000 filter
- 1999 (1) Apply 1999 filter
- 1997 (1) Apply 1997 filter
- 1995 (2) Apply 1995 filter
- 1994 (2) Apply 1994 filter
- 1993 (2) Apply 1993 filter
- 1992 (1) Apply 1992 filter
- 1991 (2) Apply 1991 filter
- 1990 (3) Apply 1990 filter
- 1989 (2) Apply 1989 filter
- 1987 (2) Apply 1987 filter
- 1986 (1) Apply 1986 filter
- 1985 (1) Apply 1985 filter
- 1984 (1) Apply 1984 filter
- 1983 (1) Apply 1983 filter
- 1982 (2) Apply 1982 filter
- 1981 (1) Apply 1981 filter
- 1979 (1) Apply 1979 filter
- 1973 (1) Apply 1973 filter
Type of Publication
139 Publications
Showing 71-80 of 139 resultsAssigning behavioral functions to neural structures has long been a central goal in neuroscience and is a necessary first step toward a circuit-level understanding of how the brain generates behavior. Here, we map the neural substrates of locomotion and social behaviors for Drosophila melanogaster using automated machine-vision and machine-learning techniques. From videos of 400,000 flies, we quantified the behavioral effects of activating 2,204 genetically targeted populations of neurons. We combined a novel quantification of anatomy with our behavioral analysis to create brain-behavior correlation maps, which are shared as browsable web pages and interactive software. Based on these maps, we generated hypotheses of regions of the brain causally related to sensory processing, locomotor control, courtship, aggression, and sleep. Our maps directly specify genetic tools to target these regions, which we used to identify a small population of neurons with a role in the control of walking. •We developed machine-vision methods to broadly and precisely quantify fly behavior•We measured effects of activating 2,204 genetically targeted neuronal populations•We created whole-brain maps of neural substrates of locomotor and social behaviors•We created resources for exploring our results and enabling further investigation Machine-vision analyses of large behavior and neuroanatomy data reveal whole-brain maps of regions associated with numerous complex behaviors.
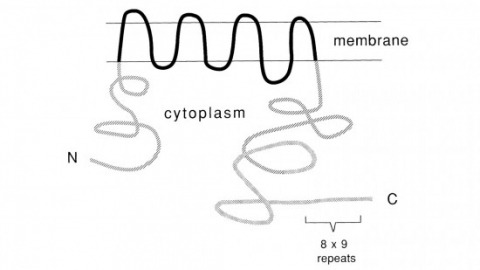
Recent studies suggest that the fly uses the inositol lipid signaling system for visual excitation and that the Drosophila transient receptor potential (trp) mutation disrupts this process subsequent to the production of IP3. In this paper, we show that trp encodes a novel 1275 amino acid protein with eight putative transmembrane segments. Immunolocalization indicates that the trp protein is expressed predominantly in the rhabdomeric membranes of the photoreceptor cells.
Insects, like most animals, tend to steer away from imminent threats [1-7]. Drosophila melanogaster, for example, generally initiate an escape take-off in response to a looming visual stimulus, mimicking a potential predator [8]. The escape response to a visual threat is, however, flexible [9-12] and can alternatively consist of walking backward away from the perceived threat [11], which may be a more effective response to ambush predators such as nymphal praying mantids [7]. Flexibility in escape behavior may also add an element of unpredictability that makes it difficult for predators to anticipate or learn the prey's likely response [3-6]. Whereas the fly's escape jump has been well studied [8, 9, 13-18], the neuronal underpinnings of evasive walking remain largely unexplored. We previously reported the identification of a cluster of descending neurons-the moonwalker descending neurons (MDNs)-the activity of which is necessary and sufficient to trigger backward walking [19], as well as a population of visual projection neurons-the lobula columnar 16 (LC16) cells-that respond to looming visual stimuli and elicit backward walking and turning [11]. Given the similarity of their activation phenotypes, we hypothesized that LC16 neurons induce backward walking via MDNs and that turning while walking backward might reflect asymmetric activation of the left and right MDNs. Here, we present data from functional imaging, behavioral epistasis, and unilateral activation experiments that support these hypotheses. We conclude that LC16 and MDNs are critical components of the neural circuit that transduces threatening visual stimuli into directional locomotor output.
How neurons form synapses within specific layers remains poorly understood. In the Drosophila medulla, neurons target to discrete layers in a precise fashion. Here we demonstrate that the targeting of L3 neurons to a specific layer occurs in two steps. Initially, L3 growth cones project to a common domain in the outer medulla, overlapping with the growth cones of other neurons destined for a different layer through the redundant functions of N-Cadherin (CadN) and Semaphorin-1a (Sema-1a). CadN mediates adhesion within the domain and Sema-1a mediates repulsion through Plexin A (PlexA) expressed in an adjacent region. Subsequently, L3 growth cones segregate from the domain into their target layer in part through Sema-1a/PlexA-dependent remodeling. Together, our results and recent studies argue that the early medulla is organized into common domains, comprising processes bound for different layers, and that discrete layers later emerge through successive interactions between processes within domains and developing layers.
Site-specific recombinases have been used for two decades to manipulate the structure of animal genomes in highly predictable ways and have become major research tools. However, the small number of recombinases demonstrated to have distinct specificities, low toxicity, and sufficient activity to drive reactions to completion in animals has been a limitation. In this report we show that four recombinases derived from yeast-KD, B2, B3, and R-are highly active and nontoxic in Drosophila and that KD, B2, B3, and the widely used FLP recombinase have distinct target specificities. We also show that the KD and B3 recombinases are active in mice.
Aversive olfactory memory is formed in the mushroom bodies in Drosophila melanogaster. Memory retrieval requires mushroom body output, but the manner in which a memory trace in the mushroom body drives conditioned avoidance of a learned odor remains unknown. To identify neurons that are involved in olfactory memory retrieval, we performed an anatomical and functional screen of defined sets of mushroom body output neurons. We found that MB-V2 neurons were essential for retrieval of both short- and long-lasting memory, but not for memory formation or memory consolidation. MB-V2 neurons are cholinergic efferent neurons that project from the mushroom body vertical lobes to the middle superiormedial protocerebrum and the lateral horn. Notably, the odor response of MB-V2 neurons was modified after conditioning. As the lateral horn has been implicated in innate responses to repellent odorants, we propose that MB-V2 neurons recruit the olfactory pathway involved in innate odor avoidance during memory retrieval.
Animals discriminate stimuli, learn their predictive value and use this knowledge to modify their behavior. In Drosophila, the mushroom body (MB) plays a key role in these processes. Sensory stimuli are sparsely represented by ∼2000 Kenyon cells, which converge onto 34 output neurons (MBONs) of 21 types. We studied the role of MBONs in several associative learning tasks and in sleep regulation, revealing the extent to which information flow is segregated into distinct channels and suggesting possible roles for the multi-layered MBON network. We also show that optogenetic activation of MBONs can, depending on cell type, induce repulsion or attraction in flies. The behavioral effects of MBON perturbation are combinatorial, suggesting that the MBON ensemble collectively represents valence. We propose that local, stimulus-specific dopaminergic modulation selectively alters the balance within the MBON network for those stimuli. Our results suggest that valence encoded by the MBON ensemble biases memory-based action selection.
Evolution has tuned the nervous system of most animals to produce stereotyped behavioural responses to ethologically relevant stimuli. For example, female Drosophila avoid laying eggs in the presence of geosmin, an odorant produced by toxic moulds. Using this system, we now identify third order olfactory neurons that are essential for an innate aversive behaviour. Connectomics data place these neurons in the context of a complete synaptic circuit from sensory input to descending output. We find multiple levels of valence-specific convergence, including a novel form of axo-axonic input onto second order neurons conveying another danger signal, the pheromone of parasitoid wasps. However we also observe a massive divergence as geosmin-responsive second order olfactory neurons connect with a diverse array of ∼75 cell types. Our data suggest a transition from a labelled line organisation in the periphery to one in which olfactory information is mapped onto many different higher order populations with distinct behavioural significance.
When navigating in their environment, animals use visual motion cues as feedback signals that are elicited by their own motion. Such signals are provided by wide-field neurons sampling motion directions at multiple image points as the animal maneuvers. Each one of these neurons responds selectively to a specific optic flow-field representing the spatial distribution of motion vectors on the retina. Here, we describe the discovery of a group of local, inhibitory interneurons in the fruit fly Drosophila key for filtering these cues. Using anatomy, molecular characterization, activity manipulation, and physiological recordings, we demonstrate that these interneurons convey direction-selective inhibition to wide-field neurons with opposite preferred direction and provide evidence for how their connectivity enables the computation required for integrating opposing motions. Our results indicate that, rather than sharpening directional selectivity per se, these circuit elements reduce noise by eliminating non-specific responses to complex visual information.
•Discovery of bi-stratified glutamatergic lobula plate-intrinsic (LPi) interneurons•LPi neurons provide visual null direction inhibition to wide-field tangential cells•Blocking LPi activity leads to target neurons responding to inadequate motion cues•Motion opponency thus increases flow-field selectivity
Newly identified inhibitory neurons are central to an integrative circuit that enables Drosophila to process visual cues with opposite motions generated during flight. The neurons are required to discriminate between distinct complex motion patterns, indicating that neural processing of opposing cues can yield outcomes beyond the simple sum of two inputs.
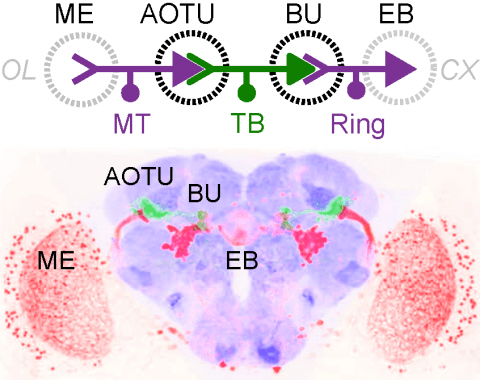
Many animals orient using visual cues, but how a single cue is selected from among many is poorly understood. Here we show that Drosophila ring neurons—central brain neurons implicated in navigation—display visual stimulus selection. Using in vivo two-color two-photon imaging with genetically encoded calcium indicators, we demonstrate that individual ring neurons inherit simple-cell-like receptive fields from their upstream partners. Stimuli in the contralateral visual field suppressed responses to ipsilateral stimuli in both populations. Suppression strength depended on when and where the contralateral stimulus was presented, an effect stronger in ring neurons than in their upstream inputs. This history-dependent effect on the temporal structure of visual responses, which was well modeled by a simple biphasic filter, may determine how visual references are selected for the fly's internal compass. Our approach highlights how two-color calcium imaging can help identify and localize the origins of sensory transformations across synaptically connected neural populations.