Recent Publications
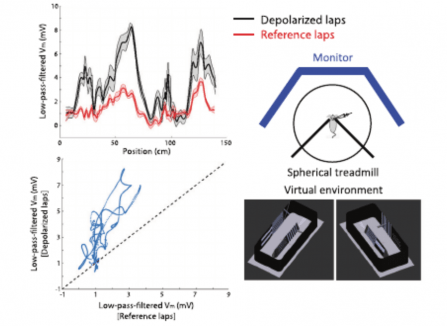
View More PublicationsTo successfully perform goal-directed navigation, animals must know where they are and what they are doing-e.g., looking for water, bringing food back to the nest, or escaping from a predator. Hippocampal neurons code for these critical variables conjunctively, but little is known about how this "where/what" code is formed or flexibly routed to other brain regions. To address these questions, we performed intracellular whole-cell recordings in mouse CA1 during a cued, two-choice virtual navigation task. We demonstrate that plateau potentials in CA1 pyramidal neurons rapidly strengthen synaptic inputs carrying conjunctive information about position and choice. Plasticity-induced response fields were modulated by cues only in animals previously trained to collect rewards based on available cues. Thus, we reveal that gradual learning is required for the formation of a conjunctive population code, upstream of CA1, while plateau-potential-induced synaptic plasticity in CA1 enables flexible routing of the code to downstream brain regions.
View Publication PageAs animals navigate, they must identify features within context. In the mammalian brain, the hippocampus has the ability to separately encode different environmental contexts, even when they share some prominent features. To do so, neurons respond to sensory features in a context-dependent manner; however, it is not known how this encoding emerges. To examine this, we performed electrical recordings in the hippocampus as mice navigated in two distinct virtual environments. In CA1, both synaptic input to single neurons and population activity strongly tracked visual cues in one environment, whereas responses were almost completely absent when the same cue was presented in a second environment. A very similar, highly context-dependent pattern of cue-driven spiking was also observed in CA3. These results indicate that CA1 inherits a complex spatial code from upstream regions, including CA3, that have already computed a context-dependent representation of environmental features.
View Publication PageNeuronal cell types are the nodes of neural circuits that determine the flow of information within the brain. Neuronal morphology, especially the shape of the axonal arbor, provides an essential descriptor of cell type and reveals how individual neurons route their output across the brain. Despite the importance of morphology, few projection neurons in the mouse brain have been reconstructed in their entirety. Here we present a robust and efficient platform for imaging and reconstructing complete neuronal morphologies, including axonal arbors that span substantial portions of the brain. We used this platform to reconstruct more than 1,000 projection neurons in the motor cortex, thalamus, subiculum, and hypothalamus. Together, the reconstructed neurons constitute more than 85 meters of axonal length and are available in a searchable online database. Axonal shapes revealed previously unknown subtypes of projection neurons and suggest organizational principles of long-range connectivity.
View Publication PageThe active properties of dendrites can support local nonlinear operations, but previous imaging and electrophysiological measurements have produced conflicting views regarding the prevalence and selectivity of local nonlinearities in vivo. We imaged calcium signals in pyramidal cell dendrites in the motor cortex of mice performing a tactile decision task. A custom microscope allowed us to image the soma and up to 300 μm of contiguous dendrite at 15 Hz, while resolving individual spines. New analysis methods were used to estimate the frequency and spatial scales of activity in dendritic branches and spines. The majority of dendritic calcium transients were coincident with global events. However, task-associated calcium signals in dendrites and spines were compartmentalized by dendritic branching and clustered within branches over approximately 10 μm. Diverse behavior-related signals were intermingled and distributed throughout the dendritic arbor, potentially supporting a large learning capacity in individual neurons.
View Publication PageThe mammalian hippocampus forms a cognitive map using neurons that fire according to an animal's position ("place cells") and many other behavioral and cognitive variables. The responses of these neurons are shaped by their presynaptic inputs and the nature of their postsynaptic integration. In CA1 pyramidal neurons, spatial responses in vivo exhibit a strikingly supralinear dependence on baseline membrane potential. The biophysical mechanisms underlying this nonlinear cellular computation are unknown. Here, through a combination of in vitro, in vivo, and in silico approaches, we show that persistent sodium current mediates the strong membrane potential dependence of place cell activity. This current operates at membrane potentials below the action potential threshold and over seconds-long timescales, mediating a powerful and rapidly reversible amplification of synaptic responses, which drives place cell firing. Thus, we identify a biophysical mechanism that shapes the coding properties of neurons composing the hippocampal cognitive map.
View Publication PageThe mammalian hippocampus, comprised of serially connected subfields, participates in diverse behavioral and cognitive functions. It has been postulated that parallel circuitry embedded within hippocampal subfields may underlie such functional diversity. We sought to identify, delineate, and manipulate this putatively parallel architecture in the dorsal subiculum, the primary output subfield of the dorsal hippocampus. Population and single-cell RNA-seq revealed that the subiculum can be divided into two spatially adjacent subregions associated with prominent differences in pyramidal cell gene expression. Pyramidal cells occupying these two regions differed in their long-range inputs, local wiring, projection targets, and electrophysiological properties. Leveraging gene-expression differences across these regions, we use genetically restricted neuronal silencing to show that these regions differentially contribute to spatial working memory. This work provides a coherent molecular-, cellular-, circuit-, and behavioral-level demonstration that the hippocampus embeds structurally and functionally dissociable streams within its serial architecture.
View Publication PageIn the hippocampus, the classical pyramidal cell type of the subiculum acts as a primary output, conveying hippocampal signals to a diverse suite of downstream regions. Accumulating evidence suggests that the subiculum pyramidal cell population may actually be comprised of discrete subclasses. Here, we investigated the extent and organizational principles governing pyramidal cell heterogeneity throughout the mouse subiculum. Using single-cell RNA-seq, we find that the subiculum pyramidal cell population can be deconstructed into eight separable subclasses. These subclasses were mapped onto abutting spatial domains, ultimately producing a complex laminar and columnar organization with heterogeneity across classical dorsal-ventral, proximal-distal, and superficial-deep axes. We further show that these transcriptomically defined subclasses correspond to differential protein products and can be associated with specific projection targets. This work deconstructs the complex landscape of subiculum pyramidal cells into spatially segregated subclasses that may be observed, controlled, and interpreted in future experiments.
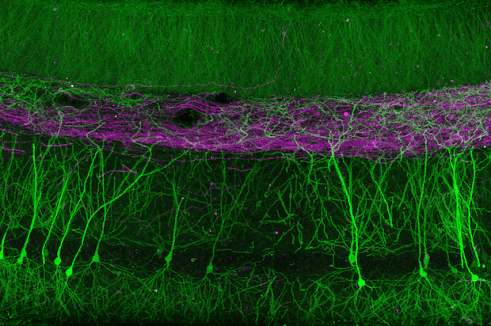
View Publication PageCA1 pyramidal neurons are a major output of the hippocampus and encode features of experience that constitute episodic memories. Feature-selective firing of these neurons results from the dendritic integration of inputs from multiple brain regions. While it is known that synchronous activation of spatially clustered inputs can contribute to firing through the generation of dendritic spikes, there is no established mechanism for spatiotemporal synaptic clustering. Here we show that single presynaptic axons form multiple, spatially clustered inputs onto the distal, but not proximal, dendrites of CA1 pyramidal neurons. These compound connections exhibit ultrastructural features indicative of strong synapses and occur much more commonly in entorhinal than in thalamic afferents. Computational simulations revealed that compound connections depolarize dendrites in a biophysically efficient manner, owing to their inherent spatiotemporal clustering. Our results suggest that distinct afferent projections use different connectivity motifs that differentially contribute to dendritic integration.
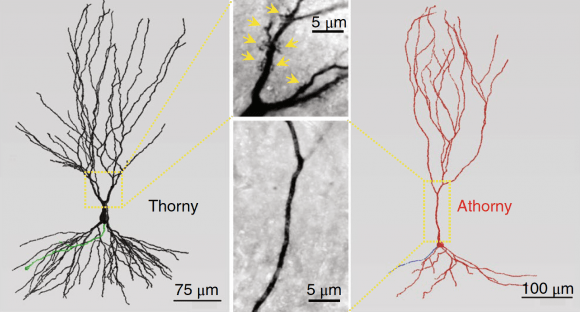
View Publication PageTo support cognitive function, the CA3 region of the hippocampus performs computations involving attractor dynamics. Understanding how cellular and ensemble activities of CA3 neurons enable computation is critical for elucidating the neural correlates of cognition. Here we show that CA3 comprises not only classically described pyramid cells with thorny excrescences, but also includes previously unidentified 'athorny' pyramid cells that lack mossy-fiber input. Moreover, the two neuron types have distinct morphological and physiological phenotypes and are differentially modulated by acetylcholine. To understand the contribution of these athorny pyramid neurons to circuit function, we measured cell-type-specific firing patterns during sharp-wave synchronization events in vivo and recapitulated these dynamics with an attractor network model comprising two principal cell types. Our data and simulations reveal a key role for athorny cell bursting in the initiation of sharp waves: transient network attractor states that signify the execution of pattern completion computations vital to cognitive function.
View Publication PageClarifying gene expression in narrowly defined neuronal populations can provide insight into cellular identity, computation, and functionality. Here, we used next-generation RNA sequencing (RNA-seq) to produce a quantitative, whole genome characterization of gene expression for the major excitatory neuronal classes of the hippocampus; namely, granule cells and mossy cells of the dentate gyrus, and pyramidal cells of areas CA3, CA2, and CA1. Moreover, for the canonical cell classes of the trisynaptic loop, we profiled transcriptomes at both dorsal and ventral poles, producing a cell-class- and region-specific transcriptional description for these populations. This dataset clarifies the transcriptional properties and identities of lesser-known cell classes, and moreover reveals unexpected variation in the trisynaptic loop across the dorsal-ventral axis. We have created a public resource, Hipposeq (http://hipposeq.janelia.org), which provides analysis and visualization of these data and will act as a roadmap relating molecules to cells, circuits, and computation in the hippocampus.
View Publication PageTissue and organ function has been conventionally understood in terms of the interactions among discrete and homogeneous cell types. This approach has proven difficult in neuroscience due to the marked diversity across different neuron classes, but it may be further hampered by prominent within-class variability. Here, we considered a well-defined canonical neuronal population-hippocampal CA1 pyramidal cells (CA1 PCs)-and systematically examined the extent and spatial rules of transcriptional heterogeneity. Using next-generation RNA sequencing, we identified striking variability in CA1 PCs, such that the differences within CA1 along the dorsal-ventral axis rivaled differences across distinct pyramidal neuron classes. This variability emerged from a spectrum of continuous gene-expression gradients, producing a transcriptional profile consistent with a multifarious continuum of cells. This work reveals an unexpected amount of variability within a canonical and narrowly defined neuronal population and suggests that continuous, within-class heterogeneity may be an important feature of neural circuits.
View Publication PageNeuronal circuit function is governed by precise patterns of connectivity between specialized groups of neurons. The diversity of GABAergic interneurons is a hallmark of cortical circuits, yet little is known about their targeting to individual postsynaptic dendrites. We examined synaptic connectivity between molecularly defined inhibitory interneurons and CA1 pyramidal cell dendrites using correlative light-electron microscopy and large-volume array tomography. We show that interneurons can be highly selective in their connectivity to specific dendritic branch types and, furthermore, exhibit precisely targeted connectivity to the origin or end of individual branches. Computational simulations indicate that the observed subcellular targeting enables control over the nonlinear integration of synaptic input or the initiation and backpropagation of action potentials in a branch-selective manner. Our results demonstrate that connectivity between interneurons and pyramidal cell dendrites is more precise and spatially segregated than previously appreciated, which may be a critical determinant of how inhibition shapes dendritic computation.
View Publication Page